Sequence-specific targeting of MSL complex regulates transcription of the roX RNA genes
- PMID: 15229655
- PMCID: PMC514957
- DOI: 10.1038/sj.emboj.7600299
Sequence-specific targeting of MSL complex regulates transcription of the roX RNA genes
Abstract
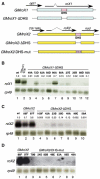
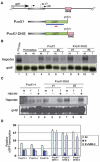
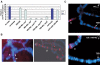
In Drosophila, dosage compensation is controlled by the male-specific lethal (MSL) complex consisting of at least five proteins and two noncoding RNAs, roX1 and roX2. The roX RNAs function in targeting MSL complex to the X chromosome, and roX transgenes can nucleate spreading of the MSL complex into flanking chromatin when inserted on an autosome. An MSL-binding site (DHS, DNaseI hypersensitive site) has been identified in each roX gene. Here, we investigate the functions of the DHS using transgenic deletion analyses and reporter assays. We find that MSL interaction with the DHS counteracts constitutive repression at roX1, resulting in male-specific expression of roX1 RNA. Surprisingly, the DHS is not required for initiation of cis spreading of MSL complex, instead local transcription of roX RNAs correlates with extensive spreading.
Figures



References
-
- Akhtar A, Becker PB (2000) Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol Cell 5: 367–375 - PubMed
-
- Amrein H, Axel R (1997) Genes expressed in neurons of adult male Drosophila. Cell 88: 459–469 - PubMed
-
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120–124 - PubMed
-
- Barolo S, Carver LA, Posakony JW (2000) GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques 29: 726–732 - PubMed
-
- Bashaw GJ, Baker BS (1997) The regulation of the Drosophila msl-2 gene reveals a function for Sex-lethal in translational control. Cell 89: 789–798 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

