Multi-dimensional time-correlated single photon counting (TCSPC) fluorescence lifetime imaging microscopy (FLIM) to detect FRET in cells
- PMID: 15230870
- PMCID: PMC1903372
- DOI: 10.1111/j.0022-2720.2004.01343.x
Multi-dimensional time-correlated single photon counting (TCSPC) fluorescence lifetime imaging microscopy (FLIM) to detect FRET in cells
Abstract
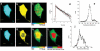
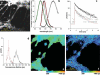
We present a novel, multi-dimensional, time-correlated single photon counting (TCSPC) technique to perform fluorescence lifetime imaging with a laser-scanning microscope operated at a pixel dwell-time in the microsecond range. The unsurpassed temporal accuracy of this approach combined with a high detection efficiency was applied to measure the fluorescent lifetimes of enhanced cyan fluorescent protein (ECFP) in isolation and in tandem with EYFP (enhanced yellow fluorescent protein). This technique enables multi-exponential decay analysis in a scanning microscope with high intrinsic time resolution, accuracy and counting efficiency, particularly at the low excitation levels required to maintain cell viability and avoid photobleaching. Using a construct encoding the two fluorescent proteins separated by a fixed-distance amino acid spacer, we were able to measure the fluorescence resonance energy transfer (FRET) efficiency determined by the interchromophore distance. These data revealed that ECFP exhibits complex exponential fluorescence decays under both FRET and non-FRET conditions, as previously reported. Two approaches to calculate the distance between donor and acceptor from the lifetime delivered values within a 10% error range. To confirm that this method can be used also to quantify intermolecular FRET, we labelled cultured neurones with the styryl dye FM1-43, quantified the fluorescence lifetime, then quenched its fluorescence using FM4-64, an efficient energy acceptor for FM1-43 emission. These experiments confirmed directly for the first time that FRET occurs between these two chromophores, characterized the lifetimes of these probes, determined the interchromophore distance in the plasma membrane and provided high-resolution two-dimensional images of lifetime distributions in living neurones.
Figures



References
-
- Bastiaens PI, Squire A. Fluorescence lifetime imaging microscopy: spatial resolution of biochemical processes in the cell. Trends Cell Biol. 1999;9:48–52. - PubMed
-
- Behne MJ, Meyer JW, Hanson KM, Barry NP, Murata S, Crumrine D, Clegg RW, Gratton E, Holleran WM, Elias PM, Mauro TM. NHE1 regulates the stratum corneum permeability barrier homeostasis. Microenvironment acidification assessed with fluorescence lifetime imaging. J. Biol. Chem. 2002;277:47399–47406. - PubMed
-
- Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. - PubMed
-
- Bevington PR. Data Reduction and Error Analysis for the Physical Sciences. New York: McGraw-Hill; 1969.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
