The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effects
- PMID: 15231642
- PMCID: PMC1575061
- DOI: 10.1038/sj.bjp.0705828
The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effects
Abstract
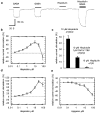
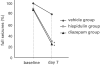
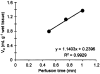
The functional characterization of hispidulin (4',5,7-trihydroxy-6-methoxyflavone), a potent benzodiazepine (BZD) receptor ligand, was initiated to determine its potential as a modulator of central nervous system activity. After chemical synthesis, hispidulin was investigated at recombinant GABA(A)/BZD receptors expressed by Xenopus laevis oocytes. Concentrations of 50 nm and higher stimulated the GABA-induced chloride currents at tested receptor subtypes (alpha(1-3,5,6)beta(2)gamma(2)S) indicating positive allosteric properties. Maximal stimulation at alpha(1)beta(2)gamma(2)S was observed with 10 microm hispidulin. In contrast to diazepam, hispidulin modulated the alpha(6)beta(2)gamma(2)S-GABA(A) receptor subtype. When fed to seizure-prone Mongolian gerbils (Meriones unguiculatus) in a model of epilepsy, hispidulin (10 mg kg(-1) body weight (BW) per day) and diazepam (2 mg kg(-1) BW per day) markedly reduced the number of animals suffering from seizures after 7 days of treatment (30 and 25% of animals in the respective treatment groups, vs 80% in the vehicle group). Permeability across the blood-brain barrier for the chemically synthesized, (14)C-labelled hispidulin was confirmed by a rat in situ perfusion model. With an uptake rate (K(in)) of 1.14 ml min(-1) g(-1), measurements approached the values obtained with highly penetrating compounds such as diazepam. Experiments with Caco-2 cells predict that orally administered hispidulin enters circulation in its intact form. At a concentration of 30 microm, the flavone crossed the monolayer without degradation as verified by the absence of glucuronidated metabolites.
Figures

Comment in
-
Flavonoids: some of the wisdom of sage?Br J Pharmacol. 2004 Jul;142(5):809-10. doi: 10.1038/sj.bjp.0705827. Br J Pharmacol. 2004. PMID: 15231641 Free PMC article. No abstract available.
References
-
- AVALLONE R., ZANOLI P., PUIA G., KLEINSCHNITZ M., SCHREIER P., BARALDI M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol. 2000;59:1387–1394. - PubMed
-
- BAKER W., BROWN N.C., SCOTT J.A. The synthesis of 5-hydroxy-8-methoxyflavone (primetin monomethyl ether) J. Chem. Soc. 1939;2:1922–1927.
-
- BERTORELLI R., ADAMI M., ONGINI E. The Mongolian gerbil in experimental epilepsy. Ital. J. Neurol. Sci. 1995;16:101–106. - PubMed
-
- BOILEAU A.J., BAUR R., SHARKEY L.M., SIGEL E., CZAJKOWSKI C. The relative amount of cRNA coding for γ2 subunits affects stimulation by benzodiazepines in GABAA receptors expressed in Xenopus oocytes. Neuropharmacology. 2002;43:695–700. - PubMed
-
- BRACON F., GUITTARD F., TAFFIN DE GIVENCHY E., CAMBON A. Highly fluorinated monomeres precursors of side-chain liquid cristalline polysiloxanes. J. Polym. Sci. A. 1999;37:4487–4496.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

