POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex
- PMID: 15231715
- PMCID: PMC478187
- DOI: 10.1101/gad.1215404
POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex
Abstract
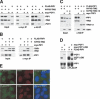
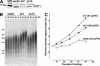
Human telomere length is controlled by a negative feedback loop based on the binding of TRF1 to double-stranded telomeric DNA. The TRF1 complex recruits POT1, a single-stranded telomeric DNA-binding protein necessary for cis-inhibition of telomerase. By mass spectrometry, we have identified a new telomeric protein, which we have named POT1-interacting protein 1 (PIP1). PIP1 bound both POT1 and the TRF1-interacting factor TIN2 and could tether POT1 to the TRF1 complex. Reduction of PIP1 or POT1 levels with shRNAs led to telomere elongation, indicating that PIP1 contributes to telomere length control through recruitment of POT1.
Copyright 2004 Cold Spring Harbor Laboratory Press ISSN
Figures


References
-
- Ancelin K., Brunori, M., Bauwens, S., Koering, C.E., Brun, C., Ricoul, M., Pommier, J.P., Sabatier, L., and Gilson, E. 2002. Targeting assay to study the cis functions of human telomeric proteins: Evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol. Cell. Biol. 22: 3474-3487. - PMC - PubMed
-
- Baumann P. and Cech, T.R. 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171-1175. - PubMed
-
- Chong L., van Steensel, B., Broccoli, D., Erdjument-Bromage, H., Hanish, J., Tempst, P., and de Lange, T. 1995. A human telomeric protein. Science 270: 1663-1667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
