Dynamic patterns of subcellular protein localization during spore coat morphogenesis in Bacillus subtilis
- PMID: 15231775
- PMCID: PMC438564
- DOI: 10.1128/JB.186.14.4441-4448.2004
Dynamic patterns of subcellular protein localization during spore coat morphogenesis in Bacillus subtilis
Abstract
Endospores of Bacillus subtilis are encased in a thick, proteinaceous shell known as the coat, which is composed of a large number of different proteins. Here we report the identification of three previously uncharacterized coat-associated proteins, YabP, YheD, and YutH, and their patterns of subcellular localization during the process of sporulation, obtained by using fusions of the proteins to the green fluorescent protein (GFP). YabP-GFP was found to form both a shell and a ring around the center of the forespore across the short axis of the sporangium. YheD-GFP, in contrast, formed two rings around the forespore that were offset from its midpoint, before it eventually redistributed to form a shell around the developing spore. Finally, YutH-GFP initially localized to a focus at one end of the forespore, which then underwent transformation into a ring that was located adjacent to the forespore. Next, the ring became a cap at the mother cell pole of the forespore that eventually spread around the entire developing spore. Thus, each protein exhibited its own distinct pattern of subcellular localization during the course of coat morphogenesis. We concluded that spore coat assembly is a dynamic process involving diverse patterns of protein assembly and localization.
Copyright 2004 American Society for Microbiology
Figures



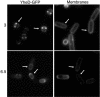
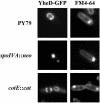


Comment in
-
From rings to layers: surprising patterns of protein deposition during bacterial spore assembly.J Bacteriol. 2004 Jul;186(14):4423-6. doi: 10.1128/JB.186.14.4423-4426.2004. J Bacteriol. 2004. PMID: 15231773 Free PMC article. No abstract available.
Similar articles
-
Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis.Genes Dev. 1994 Jan;8(2):234-44. doi: 10.1101/gad.8.2.234. Genes Dev. 1994. PMID: 8299942
-
Localization of proteins to different layers and regions of Bacillus subtilis spore coats.J Bacteriol. 2010 Jan;192(2):518-24. doi: 10.1128/JB.01103-09. Epub 2009 Nov 20. J Bacteriol. 2010. PMID: 19933362 Free PMC article.
-
Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum.Mol Microbiol. 2005 Mar;55(6):1767-81. doi: 10.1111/j.1365-2958.2005.04501.x. Mol Microbiol. 2005. PMID: 15752199
-
The Bacillus subtilis endospore: assembly and functions of the multilayered coat.Nat Rev Microbiol. 2013 Jan;11(1):33-44. doi: 10.1038/nrmicro2921. Epub 2012 Dec 3. Nat Rev Microbiol. 2013. PMID: 23202530 Free PMC article. Review.
-
[Identification and characterization of the outermost layer of Bacillus subtilis spores].Yakugaku Zasshi. 2012;132(8):919-24. doi: 10.1248/yakushi.132.919. Yakugaku Zasshi. 2012. PMID: 22864350 Review. Japanese.
Cited by
-
Genomics, evolution, and crystal structure of a new family of bacterial spore kinases.Proteins. 2010 May 1;78(6):1470-82. doi: 10.1002/prot.22663. Proteins. 2010. PMID: 20077512 Free PMC article.
-
The Influence of Sporulation Conditions on the Spore Coat Protein Composition of Bacillus subtilis Spores.Front Microbiol. 2016 Oct 13;7:1636. doi: 10.3389/fmicb.2016.01636. eCollection 2016. Front Microbiol. 2016. PMID: 27790212 Free PMC article.
-
Bacillus subtilis spores displaying RBD domain of SARS-CoV-2 spike protein.Comput Struct Biotechnol J. 2023;21:1550-1556. doi: 10.1016/j.csbj.2023.02.007. Epub 2023 Feb 8. Comput Struct Biotechnol J. 2023. PMID: 36778063 Free PMC article.
-
The genomic basis for the evolution of a novel form of cellular reproduction in the bacterium Epulopiscium.BMC Genomics. 2012 Jun 21;13:265. doi: 10.1186/1471-2164-13-265. BMC Genomics. 2012. PMID: 22721417 Free PMC article.
-
The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis.PLoS Biol. 2004 Oct;2(10):e328. doi: 10.1371/journal.pbio.0020328. Epub 2004 Sep 21. PLoS Biol. 2004. PMID: 15383836 Free PMC article.
References
-
- Abe, A., H. Koide, T. Kohne, and K. Watabe. 1995. A Bacillus subtilis spore coat polypeptide gene, cotS. Microbiology 141:1433-1454. - PubMed
-
- Asai, K., H. Takamatsu, M. Iwano, T. Kodama, K. Watabe, and N. Ogasawara. 2001. The Bacillus subtilis yabQ gene is essential for formation of the spore cortex. Microbiology 147:919-927. - PubMed
-
- Driks, A. 2002. Proteins of the spore core and coat, p. 527-536. In A. Sonenshein, J. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives, ASM Press, Washington, D.C.
-
- Driks, A., S. Roels, B. Beall, C. P. Moran, and R. Losick. 1994. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev. 8:234-244. - PubMed
-
- Eichenberger, P., S. Jensen, E. Conlon, C. van Ooij, J. Silvaggi, J. Gonzalez-Pastor, M. Fujita, S. Ben-Yehuda, P. Stragier, J. Liu, and R. Losick. 2003. The sigma E regulon and the identification of additional sporulation genes in Bacillus subtilis. J. Mol. Biol. 327:945-972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

