The CorA Mg2+ transporter is a homotetramer
- PMID: 15231793
- PMCID: PMC438605
- DOI: 10.1128/JB.186.14.4605-4612.2004
The CorA Mg2+ transporter is a homotetramer
Abstract
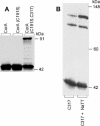
CorA is a primary Mg2+ transporter for Bacteria and Archaea. The C-terminal domain of approximately 80 amino acids forms three transmembrane (TM) segments, which suggests that CorA is a homo-oligomer. A Cys residue was added to the cytoplasmic C terminus (C317) of Salmonella enterica serovar Typhimurium CorA with or without mutation of the single periplasmic Cys191 to Ser; each mutant retained function. Oxidation of the Cys191Ser Cys317 CorA gave a dimer. Oxidation of Cys317 CorA showed a dimer plus an additional band, apparently cross-linked via both Cys317 and C191. To determine oligomer order, intact cells or purified membranes were treated with formaldehyde or carbon disulfide. Higher-molecular-mass bands formed, consistent with the presence of a tetramer. Cross-linking of the Bacillus subtilis CorA expressed in Salmonella serovar Typhimurium similarly indicated a tetramer. CorA periplasmic soluble domains from both Salmonella serovar Typhimurium and the archaeon Methanococcus jannaschii were purified and shown to retain structure. Formaldehyde treatment showed formation of a tetramer. Finally, previous mutagenesis of the CorA membrane domain identified six intramembrane residues forming an apparent pore that interacts with Mg2+ during transport. Each was mutated to Cys. In mutants carrying a single intramembrane Cys residue, spontaneous disulfide bond formation that was enhanced by oxidation with Cu(II)-1,10-phenanthroline was observed between monomers, indicating that these Mg2+-interacting residues within the membrane are very close to their cognate residue on another monomer. Thus, CorA appears to be a homotetramer with a TM segment of one monomer physically close to the same TM segment of another monomer.
Copyright 2004 American Society for Microbiology
Figures
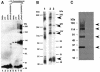

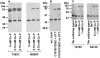
Similar articles
-
The CorA magnesium transporter gene family.Microb Comp Genomics. 1998;3(3):151-69. doi: 10.1089/omi.1.1998.3.151. Microb Comp Genomics. 1998. PMID: 9775386
-
The CorA Mg2+ transport protein of Salmonella typhimurium. Mutagenesis of conserved residues in the third membrane domain identifies a Mg2+ pore.J Biol Chem. 1998 Oct 30;273(44):28663-9. doi: 10.1074/jbc.273.44.28663. J Biol Chem. 1998. PMID: 9786860
-
Cation selectivity by the CorA Mg2+ channel requires a fully hydrated cation.Biochemistry. 2010 Jul 27;49(29):5998-6008. doi: 10.1021/bi1005656. Biochemistry. 2010. PMID: 20568735 Free PMC article.
-
Magnesium transporters: properties, regulation and structure.Front Biosci. 2006 Sep 1;11:3149-63. doi: 10.2741/2039. Front Biosci. 2006. PMID: 16720382 Review.
-
Microbial magnesium transport: unusual transporters searching for identity.Mol Microbiol. 1998 Apr;28(2):217-26. doi: 10.1046/j.1365-2958.1998.00810.x. Mol Microbiol. 1998. PMID: 9622348 Review.
Cited by
-
Alternative σ Factors Regulate Overlapping as Well as Distinct Stress Response and Metabolic Functions in Listeria monocytogenes under Stationary Phase Stress Condition.Pathogens. 2021 Apr 1;10(4):411. doi: 10.3390/pathogens10040411. Pathogens. 2021. PMID: 33915780 Free PMC article.
-
Nickel resistance in fission yeast associated with the magnesium transport system.Mol Biotechnol. 2006 Feb;32(2):139-46. doi: 10.1385/MB:32:2:139. Mol Biotechnol. 2006. PMID: 16444015
-
Adaptive response of Group B streptococcus to high glucose conditions: new insights on the CovRS regulation network.PLoS One. 2013 Apr 9;8(4):e61294. doi: 10.1371/journal.pone.0061294. Print 2013. PLoS One. 2013. PMID: 23585887 Free PMC article.
-
CorA affects tolerance of Escherichia coli and Salmonella enterica serovar Typhimurium to the lactoperoxidase enzyme system but not to other forms of oxidative stress.Appl Environ Microbiol. 2005 Nov;71(11):6515-23. doi: 10.1128/AEM.71.11.6515-6523.2005. Appl Environ Microbiol. 2005. PMID: 16269676 Free PMC article.
-
Leaf cDNA-AFLP analysis of two citrus species differing in manganese tolerance in response to long-term manganese-toxicity.BMC Genomics. 2013 Sep 14;14:621. doi: 10.1186/1471-2164-14-621. BMC Genomics. 2013. PMID: 24034812 Free PMC article.
References
-
- Chang, G., R. H. Spencer, A. T. Lee, M. T. Barclay, and D. C. Rees. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282:2220-2226. - PubMed
-
- Chou, P. Y., and G. D. Fasman. 1974. Prediction of protein conformation. Biochemistry 13:222-245. - PubMed
-
- Falke, J. J., and D. E. Koshland, Jr. 1987. Global flexibility in a sensory receptor: a site-directed cross-linking approach. Science 237:1596-1600. - PubMed
-
- Garcia, V. E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. - PubMed
-
- Garnier, J., J. F. Gibrat, and B. Robson. 1996. GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol. 266:540-553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

