Contiguous stereogenic quaternary carbons: a daunting challenge in natural products synthesis
- PMID: 15232003
- PMCID: PMC514413
- DOI: 10.1073/pnas.0402416101
Contiguous stereogenic quaternary carbons: a daunting challenge in natural products synthesis
Abstract
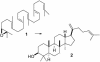
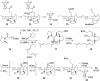
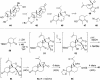
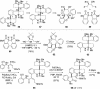
One element of structure that invariably increases the difficulty of a chemical synthesis is the presence in the target molecule of contiguous all-carbon quaternary stereocenters. This Perspective will examine the most useful transformations and strategies devised recently for directly assembling this structural unit.
Figures
Similar articles
-
Navigating the Pauson-Khand Reaction in Total Syntheses of Complex Natural Products.Acc Chem Res. 2021 Feb 2;54(3):556-568. doi: 10.1021/acs.accounts.0c00709. Epub 2021 Jan 7. Acc Chem Res. 2021. PMID: 33412841
-
Total synthesis of (+/-)-halichlorine, (+/-)-pinnaic acid, and (+/-)-tauropinnaic acid.Proc Natl Acad Sci U S A. 2004 Aug 17;101(33):12079-84. doi: 10.1073/pnas.0403887101. Epub 2004 Aug 6. Proc Natl Acad Sci U S A. 2004. PMID: 15299146 Free PMC article.
-
Synthetic Strategies toward Natural Products Containing Contiguous Stereogenic Quaternary Carbon Atoms.Angew Chem Int Ed Engl. 2016 Mar 18;55(13):4156-86. doi: 10.1002/anie.201507549. Epub 2016 Feb 2. Angew Chem Int Ed Engl. 2016. PMID: 26836448 Free PMC article. Review.
-
New Strategies in the Efficient Total Syntheses of Polycyclic Natural Products.Acc Chem Res. 2020 Nov 17;53(11):2569-2586. doi: 10.1021/acs.accounts.0c00531. Epub 2020 Nov 2. Acc Chem Res. 2020. PMID: 33136373
-
Direct construction of vicinal all-carbon quaternary stereocenters in natural product synthesis.Nat Prod Rep. 2015 Nov;32(11):1584-601. doi: 10.1039/c5np00046g. Epub 2015 Sep 3. Nat Prod Rep. 2015. PMID: 26334685 Review.
Cited by
-
Formation of Pyrroloindolines via the Alkylation of Tryptamines with Trichloroacetimidates.Tetrahedron Lett. 2021 Aug 3;77:153256. doi: 10.1016/j.tetlet.2021.153256. Epub 2021 Jul 1. Tetrahedron Lett. 2021. PMID: 34334833 Free PMC article.
-
Welwistatin support studies: expansion and limitation of aryllead(IV) coupling reactions.J Org Chem. 2007 Aug 31;72(18):6885-90. doi: 10.1021/jo071156l. Epub 2007 Aug 9. J Org Chem. 2007. PMID: 17685656 Free PMC article.
-
Diastereospecific nazarov cyclization of fully substituted dienones: generation of vicinal all-carbon-atom quaternary stereocenters.Angew Chem Int Ed Engl. 2013 Oct 11;52(42):11102-5. doi: 10.1002/anie.201305218. Epub 2013 Sep 3. Angew Chem Int Ed Engl. 2013. PMID: 24002922 Free PMC article.
-
Congested C-C bonds by Pd-catalyzed enantioselective allyl-allyl cross-coupling, a mechanism-guided solution.J Am Chem Soc. 2014 May 14;136(19):7092-100. doi: 10.1021/ja502280w. Epub 2014 Apr 29. J Am Chem Soc. 2014. PMID: 24720611 Free PMC article.
-
Scalable Synthesis of Esp and Rhodium(II) Carboxylates from Acetylacetone and RhCl3·xH2O.Org Process Res Dev. 2020 Jun 19;24(6):1207-1212. doi: 10.1021/acs.oprd.0c00164. Epub 2020 Apr 14. Org Process Res Dev. 2020. PMID: 32587455 Free PMC article.
References
-
- Johnson, W. S. (1976) Angew. Chem. Int. Ed. Engl. 15, 9-17. - PubMed
-
- Stork, G. & Burgstahler, A. W. (1955) J. Am. Chem. Soc. 77, 5068-5077.
-
- Eschenmoser, A., Ruzicka, L., Jeger, O. & Arigoni, D. (1955) Helv. Chim. Acta 38, 1890-1904.
-
- Johnson, W. S., Lindell, S. D. & Steele, J. (1987) J. Am. Chem. Soc. 109, 5852-5853.
-
- Corey, E. J. & Lin, S. (1996) J. Am. Chem. Soc. 118, 8765-8766.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources