Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system
- PMID: 15232610
- PMCID: PMC437968
- DOI: 10.1172/JCI20645
Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system
Abstract
We describe a murine model of early pregnancy failure induced by systemic activation of the CD40 immune costimulatory pathway. Although fetal loss involved an NK cell intermediate, it was not due to lymphocyte-mediated destruction of the fetus and placenta. Rather, pregnancy failure resulted from impaired progesterone synthesis by the corpus luteum of the ovary, an endocrine defect in turn associated with ovarian resistance to the gonadotropic effects of prolactin. Pregnancy failure also required the proinflammatory cytokine TNF-alpha and correlated with the luteal induction of the prolactin receptor signaling inhibitors suppressor of cytokine signaling 1 (Socs1) and Socs3. Such links between immune activation and reproductive endocrine dysfunction may be relevant to pregnancy loss and other clinical disorders of reproduction.
Figures
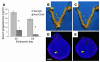
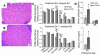
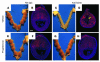
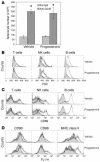

Comment in
-
A noninflammatory pathway for pregnancy loss: innate immune activation?J Clin Invest. 2004 Jul;114(1):15-7. doi: 10.1172/JCI22258. J Clin Invest. 2004. PMID: 15232605 Free PMC article. Review.
Similar articles
-
Prevention of Defective Placentation and Pregnancy Loss by Blocking Innate Immune Pathways in a Syngeneic Model of Placental Insufficiency.J Immunol. 2015 Aug 1;195(3):1129-38. doi: 10.4049/jimmunol.1402220. Epub 2015 Jun 12. J Immunol. 2015. PMID: 26071558 Free PMC article.
-
Corpus luteum development: lessons from genetic models in mice.Curr Top Dev Biol. 2005;68:49-84. doi: 10.1016/S0070-2153(05)68003-9. Curr Top Dev Biol. 2005. PMID: 16124996 Review.
-
A noninflammatory pathway for pregnancy loss: innate immune activation?J Clin Invest. 2004 Jul;114(1):15-7. doi: 10.1172/JCI22258. J Clin Invest. 2004. PMID: 15232605 Free PMC article. Review.
-
Maximal expression of suppressors of cytokine signaling in the rat ovary occurs in late pregnancy.Reproduction. 2009 Sep;138(3):537-44. doi: 10.1530/REP-08-0425. Epub 2009 Jun 5. Reproduction. 2009. PMID: 19502454
-
Prostaglandin F(2alpha) (PGF(2alpha)) and prolactin signaling: PGF(2alpha)-mediated inhibition of prolactin receptor expression in the Corpus luteum.Endocrinology. 2003 Aug;144(8):3301-5. doi: 10.1210/en.2003-0420. Endocrinology. 2003. PMID: 12865306
Cited by
-
Progesterone and HMOX-1 promote fetal growth by CD8+ T cell modulation.J Clin Invest. 2015 Apr;125(4):1726-38. doi: 10.1172/JCI68140. Epub 2015 Mar 16. J Clin Invest. 2015. PMID: 25774501 Free PMC article.
-
Autoimmune Regulator is required in female mice for optimal embryonic development and implantation†.Biol Reprod. 2019 Jun 1;100(6):1492-1504. doi: 10.1093/biolre/ioz023. Biol Reprod. 2019. PMID: 30770532 Free PMC article.
-
The Risk of Subsequent Miscarriage in Pregnant Women with Prior Polycystic Ovarian Syndrome: A Nationwide Population-Based Study.Int J Environ Res Public Health. 2021 Aug 4;18(16):8253. doi: 10.3390/ijerph18168253. Int J Environ Res Public Health. 2021. PMID: 34444016 Free PMC article.
-
Regulatory T cells in pregnancy.Springer Semin Immunopathol. 2006 Aug;28(1):31-9. doi: 10.1007/s00281-006-0023-6. Epub 2006 Jul 13. Springer Semin Immunopathol. 2006. PMID: 16838178 Review.
-
Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions.J Clin Invest. 2013 Sep;123(9):4063-75. doi: 10.1172/JCI70098. Epub 2013 Aug 27. J Clin Invest. 2013. PMID: 23979163 Free PMC article.
References
-
- Laird SM, et al. A review of immune cells and molecules in women with recurrent miscarriage. Hum. Reprod. Update. 2003;9:163–174. - PubMed
-
- Aoki K, et al. Preconceptional natural-killer-cell activity as a predictor of miscarriage. Lancet. 1995;345:1340–1342. - PubMed
-
- Coulam CB, Goodman C, Roussev RG, Thomason EJ, Beaman KD. Systemic CD56+ cells can predict pregnancy outcome. Am. J. Reprod. Immunol. 1995;33:40–46. - PubMed
-
- Kwak JY, et al. Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am. J. Reprod. Immunol. 1995;34:93–99. - PubMed
-
- Emmer PM, et al. Peripheral natural killer cytotoxicity and CD56+ CD16+ cells increase during early pregnancy in women with a history of recurrent spontaneous abortion. Hum. Reprod. 2000;15:1163–1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

