PPARalpha governs glycerol metabolism
- PMID: 15232616
- PMCID: PMC437964
- DOI: 10.1172/JCI20468
PPARalpha governs glycerol metabolism
Abstract
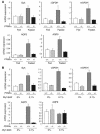
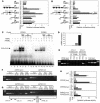
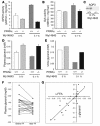
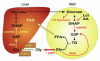
Glycerol, a product of adipose tissue lipolysis, is an important substrate for hepatic glucose synthesis. However, little is known about the regulation of hepatic glycerol metabolism. Here we show that several genes involved in the hepatic metabolism of glycerol, i.e., cytosolic and mitochondrial glycerol 3-phosphate dehydrogenase (GPDH), glycerol kinase, and glycerol transporters aquaporin 3 and 9, are upregulated by fasting in wild-type mice but not in mice lacking PPARalpha. Furthermore, expression of these genes was induced by the PPARalpha agonist Wy14643 in wild-type but not PPARalpha-null mice. In adipocytes, which express high levels of PPARgamma, expression of cytosolic GPDH was enhanced by PPARgamma and beta/delta agonists, while expression was decreased in PPARgamma(+/-) and PPARbeta/delta(-/-) mice. Transactivation, gel shift, and chromatin immunoprecipitation experiments demonstrated that cytosolic GPDH is a direct PPAR target gene. In line with a stimulating role of PPARalpha in hepatic glycerol utilization, administration of synthetic PPARalpha agonists in mice and humans decreased plasma glycerol. Finally, hepatic glucose production was decreased in PPARalpha-null mice simultaneously fasted and exposed to Wy14643, suggesting that the stimulatory effect of PPARalpha on gluconeogenic gene expression was translated at the functional level. Overall, these data indicate that PPARalpha directly governs glycerol metabolism in liver, whereas PPARgamma regulates glycerol metabolism in adipose tissue.
Figures



Similar articles
-
The G0/G1 switch gene 2 is a novel PPAR target gene.Biochem J. 2005 Dec 1;392(Pt 2):313-24. doi: 10.1042/BJ20050636. Biochem J. 2005. PMID: 16086669 Free PMC article.
-
PPARalpha agonists reduce 11beta-hydroxysteroid dehydrogenase type 1 in the liver.Biochem Biophys Res Commun. 2000 Dec 20;279(2):330-6. doi: 10.1006/bbrc.2000.3966. Biochem Biophys Res Commun. 2000. PMID: 11118287
-
Peroxisome proliferator-activated receptor alpha (PPARalpha) influences substrate utilization for hepatic glucose production.J Biol Chem. 2002 Dec 27;277(52):50237-44. doi: 10.1074/jbc.M201208200. Epub 2002 Aug 9. J Biol Chem. 2002. PMID: 12176975
-
The Interleukin-1 receptor antagonist is a direct target gene of PPARalpha in liver.J Hepatol. 2007 May;46(5):869-77. doi: 10.1016/j.jhep.2006.11.019. Epub 2006 Dec 26. J Hepatol. 2007. PMID: 17321000
-
[PPARs activators and regulation of lipid metabolism, vascular inflammation and atherosclerosis].Journ Annu Diabetol Hotel Dieu. 2001:23-32. Journ Annu Diabetol Hotel Dieu. 2001. PMID: 11565466 Review. French. No abstract available.
Cited by
-
Hepatic glucose production pathways after three days of a high-fat diet.Metabolism. 2013 Jan;62(1):152-62. doi: 10.1016/j.metabol.2012.07.012. Epub 2012 Sep 12. Metabolism. 2013. PMID: 22981137 Free PMC article.
-
Peroxisome proliferator-activated receptor alpha target genes.PPAR Res. 2010;2010:612089. doi: 10.1155/2010/612089. Epub 2010 Sep 26. PPAR Res. 2010. PMID: 20936127 Free PMC article.
-
Aquaglyceroporin Modulators as Emergent Pharmacological Molecules for Human Diseases.Front Mol Biosci. 2022 Feb 3;9:845237. doi: 10.3389/fmolb.2022.845237. eCollection 2022. Front Mol Biosci. 2022. PMID: 35187089 Free PMC article. Review.
-
Lifestyle and Food Habits Impact on Chronic Diseases: Roles of PPARs.Int J Mol Sci. 2019 Oct 31;20(21):5422. doi: 10.3390/ijms20215422. Int J Mol Sci. 2019. PMID: 31683535 Free PMC article. Review.
-
PPAR Alpha as a Metabolic Modulator of the Liver: Role in the Pathogenesis of Nonalcoholic Steatohepatitis (NASH).Biology (Basel). 2022 May 23;11(5):792. doi: 10.3390/biology11050792. Biology (Basel). 2022. PMID: 35625520 Free PMC article. Review.
References
-
- Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol. Metab. 2000;11:351–356. - PubMed
-
- Gonzalez-Manchon C, Martin-Requero A, Ayuso MS, Parrilla R. Role of endogenous fatty acids in the control of hepatic gluconeogenesis. Arch. Biochem. Biophys. 1992;292:95–101. - PubMed
-
- Lam TK, et al. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am. J. Physiol. Endocrinol. Metab. 2003;284:E863–E873. - PubMed
-
- Rebrin K, Steil GM, Getty L, Bergman RN. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes. 1995;44:1038–1045. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

