Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness
- PMID: 15232617
- PMCID: PMC437962
- DOI: 10.1172/JCI19569
Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness
Abstract
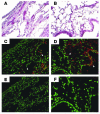
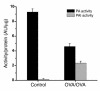
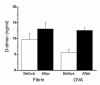
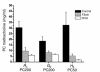
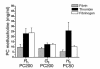
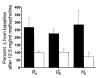
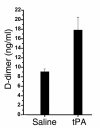
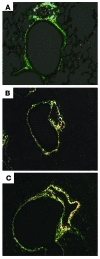
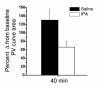
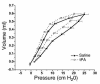
Mechanisms underlying airway hyperresponsiveness are not yet fully elucidated. One of the manifestations of airway inflammation is leakage of diverse plasma proteins into the airway lumen. They include fibrinogen and thrombin. Thrombin cleaves fibrinogen to form fibrin, a major component of thrombi. Fibrin inactivates surfactant. Surfactant on the airway surface maintains airway patency by lowering surface tension. In this study, immunohistochemically detected fibrin was seen along the luminal surface of distal airways in a patient who died of status asthmaticus and in mice with induced allergic airway inflammation. In addition, we observed altered airway fibrinolytic system protein balance consistent with promotion of fibrin deposition in mice with allergic airway inflammation. The airways of mice were exposed to aerosolized fibrinogen, thrombin, or to fibrinogen followed by thrombin. Only fibrinogen followed by thrombin resulted in airway hyperresponsiveness compared with controls. An aerosolized fibrinolytic agent, tissue-type plasminogen activator, significantly diminished airway hyperresponsiveness in mice with allergic airway inflammation. These results are consistent with the hypothesis that leakage of fibrinogen and thrombin and their accumulation on the airway surface can contribute to the pathogenesis of airway hyperresponsiveness.
Figures
Comment in
-
Coagulation-dependent mechanisms and asthma.J Clin Invest. 2004 Jul;114(1):20-3. doi: 10.1172/JCI22288. J Clin Invest. 2004. PMID: 15232607 Free PMC article. Review.
References
-
- McLane MP, et al. Interleukin-9 promotes allergen-induced eosinophilic inflammation and airway hyperresponsiveness in transgenic mice. Am. J. Respir. Cell Mol. Biol. 1998;19:713–720. - PubMed
-
- Wills-Karp M, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. - PubMed
-
- O’Byrne PM, Dolovich M, Dirks R, Roberts RS, Newhouse MT. Lung epithelial permeability: relation to nonspecific airway responsiveness. J. Appl. Physiol. 1984;57:77–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

