Developmental segregation of spinal networks driving axial- and hindlimb-based locomotion in metamorphosing Xenopus laevis
- PMID: 15235079
- PMCID: PMC1665069
- DOI: 10.1113/jphysiol.2004.069542
Developmental segregation of spinal networks driving axial- and hindlimb-based locomotion in metamorphosing Xenopus laevis
Abstract
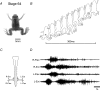
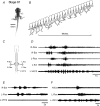
Amphibian metamorphosis includes a complete reorganization of an organism's locomotory system from axial-based swimming in larvae to limbed propulsion in the young adult. At critical stages during this behavioural switch, larval and adult motor systems operate in the same animal, commensurate with a gradual and dynamic reconfiguration of spinal locomotor circuitry. To study this plasticity, we have developed isolated preparations of the spinal cord and brainstem from pre- to post-metamorphic stages of the amphibian Xenopus laevis, in which spinal motor output patterns expressed spontaneously or in the presence of NMDA correlate with locomotor behaviour in the freely swimming animal. Extracellular ventral root recordings along the spinal cord of pre-metamorphic tadpoles revealed motor output corresponding to larval axial swimming, whereas postmetamorphic animals expressed motor patterns appropriate for bilaterally synchronous hindlimb flexion-extension kicks. However, in vitro recordings from metamorphic climax stages, with the tail and the limbs both functional, revealed two distinct motor patterns that could occur either independently or simultaneously, albeit at very different frequencies. Activity at 0.5-1 Hz in lumbar ventral roots corresponded to bipedal extension-flexion cycles, while the second, faster pattern (2-5 Hz) recorded from tail ventral roots corresponded to larval-like swimming. These data indicate that at intermediate stages during metamorphosis separate networks, one responsible for segmentally organized axial locomotion and another for more localized appendicular rhythm generation, coexist in the spinal cord and remain functional after isolation in vitro. These preparations now afford the opportunity to explore the cellular basis of locomotor network plasticity and reconfiguration necessary for behavioural changes during development.
Figures


Similar articles
-
Opposing aminergic modulation of distinct spinal locomotor circuits and their functional coupling during amphibian metamorphosis.J Neurosci. 2009 Jan 28;29(4):1163-74. doi: 10.1523/JNEUROSCI.5255-08.2009. J Neurosci. 2009. PMID: 19176825 Free PMC article.
-
Development and neuromodulation of spinal locomotor networks in the metamorphosing frog.J Physiol Paris. 2006 Nov-Dec;100(5-6):317-27. doi: 10.1016/j.jphysparis.2007.05.009. Epub 2007 Jun 8. J Physiol Paris. 2006. PMID: 17629683 Review.
-
Metamorphosis-induced changes in the coupling of spinal thoraco-lumbar motor outputs during swimming in Xenopus laevis.J Neurophysiol. 2008 Sep;100(3):1372-83. doi: 10.1152/jn.00023.2008. Epub 2008 Jul 2. J Neurophysiol. 2008. PMID: 18596184
-
Adaptive plasticity of spino-extraocular motor coupling during locomotion in metamorphosing Xenopus laevis.J Exp Biol. 2016 Apr 15;219(Pt 8):1110-21. doi: 10.1242/jeb.136168. J Exp Biol. 2016. PMID: 27103674
-
Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis.Brain Res Rev. 2008 Jan;57(1):94-102. doi: 10.1016/j.brainresrev.2007.07.018. Epub 2007 Aug 22. Brain Res Rev. 2008. PMID: 17900702 Review.
Cited by
-
Role of locomotor efference copy in vertebrate gaze stabilization.Front Neural Circuits. 2022 Dec 9;16:1040070. doi: 10.3389/fncir.2022.1040070. eCollection 2022. Front Neural Circuits. 2022. PMID: 36569798 Free PMC article. Review.
-
Characterization of spinal cord damage based on automatic video analysis of froglet swimming.Biol Open. 2019 Dec 24;8(12):bio042960. doi: 10.1242/bio.042960. Biol Open. 2019. PMID: 31852668 Free PMC article.
-
Bifurcations of Limit Cycles in a Reduced Model of the Xenopus Tadpole Central Pattern Generator.J Math Neurosci. 2018 Jul 18;8(1):10. doi: 10.1186/s13408-018-0065-9. J Math Neurosci. 2018. PMID: 30022326 Free PMC article.
-
Opposing aminergic modulation of distinct spinal locomotor circuits and their functional coupling during amphibian metamorphosis.J Neurosci. 2009 Jan 28;29(4):1163-74. doi: 10.1523/JNEUROSCI.5255-08.2009. J Neurosci. 2009. PMID: 19176825 Free PMC article.
-
Functional organization of vestibulospinal inputs responsible for tail postural control in larval Xenopus.Front Neurol. 2024 Aug 16;15:1439784. doi: 10.3389/fneur.2024.1439784. eCollection 2024. Front Neurol. 2024. PMID: 39220733 Free PMC article.
References
-
- Combes D, Merrywest SD, McLean D, Simmers J, Sillar KT. Society for Neuroscience. Washington, DC: 2002. A novel preparation for the study of neuronal plasticity during amphibian metamorphosis. Program No. 863.16. 2002 Abstract Viewer/Itinerary Planner.
-
- Combes D, Merrywest SD, Sillar KT, Simmers J. Society for Neuroscience. Washington, DC: Development of motor patterns driving limb and axial musculature recorded in vitro during amphibian metamorphosis. Program No. 277.16. 2003 Abstract Viewer/Itinerary Planner.
-
- Consoulas C, Duch C, Bayline RJ, Levine RB. Behavioral transformations during metamorphosis: remodeling of neural and motor systems. Brain Res Bull. 2000;53:571–583. - PubMed
-
- Hughes AF, Prestige MC. Development of behavior in the hindlimb of Xenopus laevis. J Zool. 1967;152:347–359.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

