Activation of Na+-H+ exchange and stretch-activated channels underlies the slow inotropic response to stretch in myocytes and muscle from the rat heart
- PMID: 15235080
- PMCID: PMC1665066
- DOI: 10.1113/jphysiol.2004.069021
Activation of Na+-H+ exchange and stretch-activated channels underlies the slow inotropic response to stretch in myocytes and muscle from the rat heart
Abstract
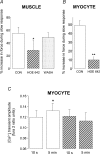
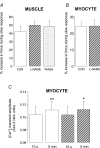
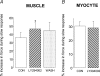
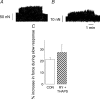
We present the first direct comparison of the major candidates proposed to underlie the slow phase of the force increase seen following myocardial stretch: (i) the Na(+)-H(+) exchanger (NHE) (ii) nitric oxide (NO) and the ryanodine receptor (RyR) and (iii) the stretch-activated channel (SAC) in both single myocytes and multicellular muscle preparations from the rat heart. Ventricular myocytes were stretched by approximately 7% using carbon fibres. Papillary muscles were stretched from 88 to 98% of the length at which maximum tension is generated (L(max)). Inhibition of NHE with HOE 642 (5 microm) significantly reduced (P < 0.05) the magnitude of the slow force response in both muscle and myocytes. Neither inhibition of phosphatidylinositol-3-OH kinase (PtdIns-3-OH kinase) with LY294002 (10 microm) nor NO synthase with L-NAME (1 mm) reduced the slow force response in muscle or myocytes (P > 0.05), and the slow response was still present in the single myocyte when the sarcoplasmic reticulum was rigorously inhibited with 1 microm ryanodine and 1 microm thapsigargin. We saw a significant reduction (P < 0.05) in the slow force response in the presence of the SAC blocker streptomycin in both muscle (80 microm) and myocytes (40 microm). In fura 2-loaded myocytes, HOE 642 and streptomycin, but not L-NAME, ablated the stretch-induced increase in [Ca(2+)](i) transient amplitude. Our data suggest that in the rat, under our experimental conditions, there are two mechanisms that underlie the slow inotropic response to stretch: activation of NHE; and of activation of SACs. Both these mechanisms are intrinsic to the myocyte.
Figures

Similar articles
-
Stretch-dependent modulation of [Na+]i, [Ca2+]i, and pHi in rabbit myocardium--a mechanism for the slow force response.Cardiovasc Res. 2005 Dec 1;68(3):454-63. doi: 10.1016/j.cardiores.2005.07.001. Epub 2005 Aug 15. Cardiovasc Res. 2005. PMID: 16099446
-
Inhibition of the Na+-H+ exchanger with cariporide abolishes stretch-induced calcium but not sodium accumulation in mouse ventricular myocytes.Cell Calcium. 2005 Jan;37(1):69-80. doi: 10.1016/j.ceca.2004.06.006. Cell Calcium. 2005. PMID: 15541465
-
Role of the Na+-H+ exchanger (NHE1) in heart muscle function during transient acidosis. A study in papillary muscles from rat and guinea pig hearts.Can J Physiol Pharmacol. 2003 Oct;81(10):937-43. doi: 10.1139/y03-091. Can J Physiol Pharmacol. 2003. PMID: 14608410
-
The slow force response to stretch in atrial and ventricular myocardium from human heart: functional relevance and subcellular mechanisms.Prog Biophys Mol Biol. 2008 Jun-Jul;97(2-3):250-67. doi: 10.1016/j.pbiomolbio.2008.02.026. Epub 2008 Mar 14. Prog Biophys Mol Biol. 2008. PMID: 18466959 Free PMC article. Review.
-
Role of stretch-activated channels on the stretch-induced changes of rat atrial myocytes.Prog Biophys Mol Biol. 2006 Jan-Apr;90(1-3):186-206. doi: 10.1016/j.pbiomolbio.2005.06.003. Epub 2005 Jul 7. Prog Biophys Mol Biol. 2006. PMID: 16043213 Review.
Cited by
-
Mechano-electric and mechano-chemo-transduction in cardiomyocytes.J Physiol. 2020 Apr;598(7):1285-1305. doi: 10.1113/JP276494. Epub 2020 Feb 3. J Physiol. 2020. PMID: 31789427 Free PMC article. Review.
-
Transmural variations in gene expression of stretch-modulated proteins in the rat left ventricle.Pflugers Arch. 2007 Jul;454(4):545-9. doi: 10.1007/s00424-007-0237-z. Epub 2007 Mar 8. Pflugers Arch. 2007. PMID: 17345093 Free PMC article.
-
Role of autocrine/paracrine mechanisms in response to myocardial strain.Pflugers Arch. 2011 Jul;462(1):29-38. doi: 10.1007/s00424-011-0930-9. Epub 2011 Feb 8. Pflugers Arch. 2011. PMID: 21301862 Review.
-
The Anrep effect in septic shock: a mechanism of cardiac adaptation.Br J Anaesth. 2025 Apr;134(4):1204-1207. doi: 10.1016/j.bja.2025.01.023. Epub 2025 Feb 17. Br J Anaesth. 2025. PMID: 39966009 Free PMC article. No abstract available.
-
Mitochondrial reactive oxygen species activate the slow force response to stretch in feline myocardium.J Physiol. 2007 Nov 1;584(Pt 3):895-905. doi: 10.1113/jphysiol.2007.141689. Epub 2007 Sep 6. J Physiol. 2007. PMID: 17823205 Free PMC article.
References
-
- Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985;17:821–840. - PubMed
-
- Alvarez BV, Perez NG, Ennis IL, Camilion de Hurtado MC, Cingolani HE. Mechanisms underlying the increase in force and Ca2+ transient that follow stretch of cardiac muscle: a possible explanation of the Anrep effect. Circ Res. 1999;85:716–722. - PubMed
-
- Belus A, White E. Effects of antibiotics on the contractility and Ca2+ transients of rat cardiac myocytes. Eur J Pharmacol. 2001;412:121–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

