Role of glial and neuronal glycine transporters in the control of glycinergic and glutamatergic synaptic transmission in lamina X of the rat spinal cord
- PMID: 15235081
- PMCID: PMC1665078
- DOI: 10.1113/jphysiol.2004.068858
Role of glial and neuronal glycine transporters in the control of glycinergic and glutamatergic synaptic transmission in lamina X of the rat spinal cord
Erratum in
- J Physiol. 2004 Sep 15;559(Pt 3):985
Abstract
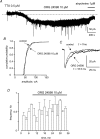
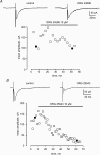
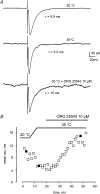
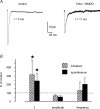
Using whole cell voltage clamp recordings from lamina X neurones in rat spinal cord slices, we investigated the effect of glycine transporter (GlyT) antagonists on both glycinergic inhibitory postsynaptic current (IPSCs) and glutamatergic excitatory postsynaptic current (EPSCs). We used ORG 24598 and ORG 25543, selective antagonists of the glial GlyT (GlyT1) and neuronal GlyT (GlyT2), respectively. In rats (P12-P16) and in the presence of kynurenic acid, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and bicuculline, ORG 24598 and ORG 25543 applied individually at a concentration of 10 microm induced a mean inward current of -10/-50 pA at -60 mV and increased significantly the decay time constants of miniature (mIPSCs), spontaneous (sIPSCs) and electrically evoked glycinergic (eIPSCs) inhibitory postsynaptic currents. ORG 25543, but not ORG 24598, decreased the frequency of mIPSCs and sIPSCs. Replacing extracellular sodium with N-methyl-d-glucamine or superfusing the slice with micromolar concentrations of glycine also increased the decay time constant of glycinergic IPSCs. By contrast, the decay time constant, amplitude and frequency of miniature GABAergic IPSCs recorded in the presence of strychnine were not affected by ORG 24598 and ORG 25543. In the presence of strychnine, bicuculline and CNQX, we recorded electrically evoked NMDA receptor-mediated EPSCs (eEPSCs). eEPSCs were suppressed by 30 micromd-2-amino-5-phosphonovalerate (APV), an antagonist of the NMDA receptor, and by 30 microm dichlorokynurenic acid (DCKA), an antagonist of the glycine site of the NMDA receptor. Glycine (1-5 microm) and d-serine (10 microm) increased the amplitude of eEPSCs whereas l-serine had no effect. ORG 24598 and ORG 25543 increased significantly the amplitude of NMDA receptor-mediated eEPSCs without affecting the amplitude of non-NMDA receptor-mediated eEPSCs. We conclude that blocking glial and/or neuronal glycine transporters increased the level of glycine in spinal cord slices, which in turn prolonged the duration of glycinergic synaptic current and potentiated the NMDA-mediated synaptic response.
Figures







References
-
- Ahmadi S, Muth-Selbach U, Lauterbach A, Lipfert P, Neuhuber WL, Zeilhofer HU. Facilitation of spinal NMDA receptor currents by spillover of synaptically released glycine. Science. 2003;300:2094–2097. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

