Purinergic regulation of guinea pig suburothelial myofibroblasts
- PMID: 15235082
- PMCID: PMC1665063
- DOI: 10.1113/jphysiol.2004.067934
Purinergic regulation of guinea pig suburothelial myofibroblasts
Abstract
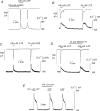
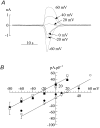
The Ca(2+)-regulating and electrophysiological properties of guinea-pig suburothelial myofibroblasts have been measured in order to investigate their potential role in the sensation of bladder fullness, due to their strategic position between the urothelium and afferent fibres. Previous work has shown that stretch of the bladder wall releases ATP. Cells that stain positively for vimentin were isolated. About 45% of cells (median membrane capacitance 13.3 pF) exhibited spontaneous depolarizations to about -25 mV with a physiological Cl(-) gradient (frequency 2.6 +/- 1.5 min(-1), duration 14.5 +/- 2.2 s, n= 15). Under voltage-clamp spontaneous inward currents (frequency 1.5 +/- 0.2 min(-1), duration 14.5 +/- 7.0 s, n= 18) were recorded, with a similar reversal potential. The spontaneous currents were preceded by intracellular Ca(2+) transients with a magnitude that was independent of membrane potential. All cells tested responded to ATP by generating an intracellular Ca(2+) transient, followed by inward currents; the currents had a similar reversal potential and slope conductance to their spontaneous counterparts. ATP-generated transients were mimicked by UTP and ADP but not by alpha,beta-methylene-ATP (1-10 microm) or CTP (30 microm), indicating that ATP acts via a P2Y receptor. Transients were partially attenuated by 1 mm suramin but PPADS (80 microm) had no effect. These data indicate that ATP acts via a P2Y receptor, but responses were resistant to the P2Y(1) antagonist MRS2179. ATP-generated transients were abolished by intracellular perfusion with heparin and TMB-8 indicating that IP(3) was the intracellular second messenger. The reversal potentials of the spontaneous and ATP-generated currents were shifted by about +45 mV by a 12-fold reduction of the extracellular [Cl(-)] and the currents were greatly attenuated by 1 mm DIDS. No transients were generated on exposure to the muscarinic agonist carbachol. We propose that these cells may play a regulatory step in the sensation of bladder fullness by responding to ATP. The precise mechanism whereby they couple urothelial ATP release to afferent excitation is the next step to be elucidated.
Figures







References
-
- Cockayne DA, Hamilton SG, Zhu Q-M, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford APD. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. - PubMed
-
- De Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Ann Rev Pharmacol Toxicol. 2001;41:691–721. - PubMed
-
- Donovan J, Naughton M, Gotoh M, Corcos J, Jackson S, Kelleher C, Lukacs B, Costa P. Symptom and quality of life assessment. In: Abrams P, Khoury S, Wein A, editors. Incontinence. Plymouth, UK: Health Publications Ltd; 1999. pp. 295–331.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

