Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices
- PMID: 15235091
- PMCID: PMC1665072
- DOI: 10.1113/jphysiol.2004.065672
Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices
Abstract
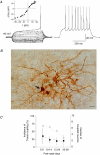
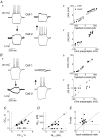
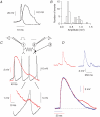
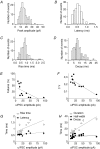
Basal ganglia are interconnected subcortical nuclei, connected to the thalamus and all cortical areas involved in sensory motor control, limbic functions and cognition. The striatal output neurones (SONs), the major striatal population, are believed to act as detectors and integrators of distributed patterns of cerebral cortex inputs. Despite the key role of SONs in cortico-striatal information processing, little is known about their local interactions. Here, we report the existence and characterization of electrical and GABAergic transmission between SONs in rat brain slices. Tracer coupling (biocytin) incidence was high during the first two postnatal weeks and then decreased (postnatal days (P) 5-25, 60%; P25-30, 29%; n= 61). Electrical coupling was observed between 27% of SON pairs (coupling coefficient: 3.1 +/- 0.3%, n= 89 at P15) and as shown by single-cell RT-PCR, several connexin (Cx) mRNAs were found to be expressed (Cx31.1, Cx32, Cx36 and Cx47). GABAergic synaptic transmission (abolished by bicuculline, a GABA(A) receptor antagonist) observed in 19% of SON pairs (n= 62) was reliable (mean failure rate of 6 +/- 3%), precise (variation coefficient of latency, 0.06), strong (IPSC amplitudes of 38 +/- 12 pA) and unidirectional. Interestingly, electrical and chemical transmission were mutually exclusive. These results suggest that preferential networks of electrically and chemically connected SONs, might be involved in the channelling of cortico-basal ganglia information processing.
Figures




References
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;7:266–271. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia. Annu Rev Neurosci. 1986;9:357–381. - PubMed
-
- Bennett MVL. Electrical transmission: a functional analysis and comparison to chemical transmission. In: Kandel ER, editor. Handbook of Physiology, section 1, The Nervous System. Vol. 1. Baltimore: Williams & Wilkins; 1977. pp. 357–416.
-
- Bennett MVL, Zukin S. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

