Changes in AMPA receptor currents following LTP induction on rat CA1 pyramidal neurones
- PMID: 15235093
- PMCID: PMC1665120
- DOI: 10.1113/jphysiol.2004.065219
Changes in AMPA receptor currents following LTP induction on rat CA1 pyramidal neurones
Abstract
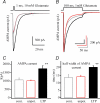
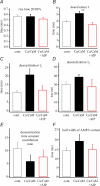
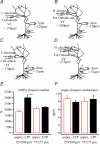
In the CA1 region of the hippocampus, LTP is thought to be initiated by a transient activation of NMDA receptors and is expressed as a persistent increase in synaptic transmission through AMPA receptors. To investigate the postsynaptic modifications of AMPA receptors involved in this enhanced synaptic transmission, the channel density and single-channel properties of extrasynaptic AMPA receptors located in synaptically active dendritic regions were examined following the induction of LTP. Following tetanic stimulation an outside-out patch was excised from the apical dendrite near the point of stimulation and saturating concentrations of glutamate were rapidly applied to the patch. AMPA current amplitude and duration were increased significantly in patches pulled from dendrites that expressed LTP. Non-stationary fluctuation analysis of AMPA currents indicated that AMPA channel number was nearly twofold larger than in controls, while single channel conductance and maximum open-probability were unchanged. Furthermore, while subtle changes in AMPA channel kinetics could also be observed, we did not find any evidence that receptor affinity or rectification properties were altered by LTP induction. Very similar results were found when CaMK-II activity was increased through the intracellular application of Ca/CaM. Together, we interpret our data to indicate that the stimuli used here produce an increased delivery of AMPA receptors to synaptically active regions of the apical dendrite without inducing any significant changes in their basic biophysical properties and that such delivery is a key element in this form of synaptic plasticity.
Figures







References
-
- Bannister NJ, Larkman AU. Dendritic morphology of CA1 pyramidal neurons from the rat hippocampus. II. Spine distribution. J Comp Neurol. 1995;360:161–171. - PubMed
-
- Benke TA, Lüthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

