Serotonin and cholecystokinin synergistically stimulate rat vagal primary afferent neurones
- PMID: 15235095
- PMCID: PMC1665123
- DOI: 10.1113/jphysiol.2004.064816
Serotonin and cholecystokinin synergistically stimulate rat vagal primary afferent neurones
Retraction in
-
Retraction: Li, Y., Wu, X. Y., & Owyang, C. (2004). Serotonin and cholecystokinin synergistically stimulate rat vagal primary afferent neurons. The Journal of Physiology, 559(2), 651-662. https://doi.org/10.1113/jphysiol.2004.064816.J Physiol. 2023 May;601(10):2045. doi: 10.1113/JP284694. Epub 2023 Apr 28. J Physiol. 2023. PMID: 36942713 No abstract available.
Abstract
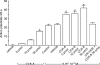
Recent studies indicate that cholecystokinin (CCK) and serotonin (5-hydroxytryptamine, 5-HT) act via vagal afferent fibres to mediate gastrointestinal functions. In the present study, we characterized the interaction between CCK and 5-HT in the vagal primary afferent neurones. Single neuronal discharges of vagal primary afferent neurones innervating the duodenum were recorded from rat nodose ganglia. Two groups of nodose ganglia neurones were identified: group A neurones responded to intra-arterial injection of low doses of cholecystokinin octapeptide (CCK-8; 10-60 pmol); group B neurones responded only to high doses of CCK-8 (120-240 pmol), and were also activated by duodenal distention. CCK-JMV-180, which acts as an agonist in high-affinity states and as an antagonist in low-affinity states, dose dependently stimulated group A neurones, but inhibited the effect of the high doses of CCK-8 on group B neurones. Duodenal perfusion of 5-HT evoked dose-dependent increases in nodose neuronal discharges. Some neurones that responded to 5-HT showed no response to either high or low doses of CCK-8. A separate group of nodose neurones that possessed high-affinity CCK type A (CCK-A) receptors also responded to luminal infusion of 5-HT. Further, a subthreshold dose of CCK-8 (i.e. 5 pmol) produced no measurable electrophysiological effects but it augmented the neuronal responses to 5-HT. This potentiation effect of CCK-8 was eliminated by CR 1409. From these results we concluded that the vagal nodose ganglion contains neurones that may possess only high- or low-affinity CCK-A receptors or 5-HT3 receptors. Some neurones that express high-affinity CCK-A receptors also express 5-HT3 receptors. Pre-exposure to luminal 5-HT may augment the subsequent response to a subthreshold dose of CCK.
Figures


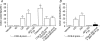
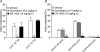

References
-
- Andrews PL, Davison JS. Activation of vagal afferent terminals by 5-HT is mediated by 5-HT3 receptors in the anaesthetized ferret. J Physiol. 1990;422:92P.
-
- Attoub S, Moizo L, Laignwau JP, Alchepo B, Lewin MJ, Bado A. YM022, a highly potent and selective CCKB antagonist inhibiting gastric acid secretion in the rat, the cat and isolated rabbit glands. Fundam Clin Pharmacol. 1998;12:256–262. - PubMed
-
- Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. J Auton Nerv Syst. 1993;45:41–50. - PubMed
-
- Bueno L, Fioramonti J, Delvaux M, Frexinos J. Mediators and pharmacology of visceral sensitivity: from basic to clinical investigations. Gastroenterology. 1997;112:1714–1743. - PubMed
-
- Cox JE. Duodenal sucrose and glucose infusions enhance suppression by cholecystokinin of sham feeding. Am J Physiol. 1994;266:R466–R471. - PubMed

