Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease
- PMID: 15235129
- PMCID: PMC478583
- DOI: 10.1073/pnas.0403342101
Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease
Abstract
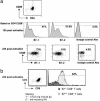
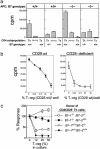
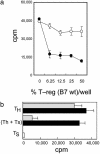
Although there is considerable evidence that a subpopulation of regulatory CD4(+)CD25+ T cells can suppress the response of autoreactive T cells, the underlying molecular mechanism is not understood. We find that transmission of a suppressive signal by CD4CD25+ regulatory cells requires engagement of the B7 molecule expressed on target T cells. The response of T cells from B7-deficient mice is resistant to suppression in vitro, and these cells provoke a lethal wasting disease in lymphopenic mice despite the presence of regulatory T cells. Susceptibility of B7-deficient cells to suppression is restored by lentiviral-based expression of full-length, but not truncated, B7 lacking a transmembrane/cytoplasmic domain. Because expression of these B7 truncation mutants restores CD28-dependent costimulatory activity, these findings that indicate B7-based transmission of suppressive activity suggest new approaches to modifying autoimmune responses.
Figures


Similar articles
-
Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo.J Immunol. 2005 Jun 1;174(11):6648-56. doi: 10.4049/jimmunol.174.11.6648. J Immunol. 2005. PMID: 15905503
-
B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes.Immunity. 2000 Apr;12(4):431-40. doi: 10.1016/s1074-7613(00)80195-8. Immunity. 2000. PMID: 10795741
-
B7-CD28 interaction promotes proliferation and survival but suppresses differentiation of CD4-CD8- T cells in the thymus.J Immunol. 2004 Aug 15;173(4):2253-61. doi: 10.4049/jimmunol.173.4.2253. J Immunol. 2004. PMID: 15294937
-
The CD28/B7 pathway: a novel regulator of plasma cell function.Adv Exp Med Biol. 2013;785:67-75. doi: 10.1007/978-1-4614-6217-0_8. Adv Exp Med Biol. 2013. PMID: 23456839 Review.
-
The CD28-B7 costimulatory pathway and its role in autoimmune disease.J Leukoc Biol. 1997 Aug;62(2):156-62. doi: 10.1002/jlb.62.2.156. J Leukoc Biol. 1997. PMID: 9261329 Review.
Cited by
-
Molecular Mechanisms of T Helper Cell Differentiation and Functional Specialization.Immune Netw. 2023 Feb 17;23(1):e4. doi: 10.4110/in.2023.23.e4. eCollection 2023 Feb. Immune Netw. 2023. PMID: 36911803 Free PMC article. Review.
-
CD8+CD28- regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice.Gastroenterology. 2006 Dec;131(6):1775-85. doi: 10.1053/j.gastro.2006.09.008. Epub 2006 Sep 8. Gastroenterology. 2006. PMID: 17087950 Free PMC article.
-
Regulatory T cells promote corneal endothelial cell survival following transplantation via interleukin-10.Am J Transplant. 2020 Feb;20(2):389-398. doi: 10.1111/ajt.15631. Epub 2019 Oct 30. Am J Transplant. 2020. PMID: 31587452 Free PMC article.
-
Dicer-dependent microRNA pathway safeguards regulatory T cell function.J Exp Med. 2008 Sep 1;205(9):1993-2004. doi: 10.1084/jem.20081062. Epub 2008 Aug 25. J Exp Med. 2008. PMID: 18725526 Free PMC article.
-
Bridging the divide: unveiling mutual immunological pathways of cancer and pregnancy.Inflamm Res. 2024 May;73(5):793-807. doi: 10.1007/s00011-024-01866-9. Epub 2024 Mar 16. Inflamm Res. 2024. PMID: 38492049 Review.
References
-
- McHugh, R. S., Shevach, E. M. & Thornton, A. M. (2001) Microb. Infect. 3, 919–927. - PubMed
-
- Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. (2002) Nat. Immunol. 3, 135–142. - PubMed
-
- Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. (2003) Nat. Immunol. 4, 330–336. - PubMed
-
- Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299, 1057–1061. - PubMed
-
- Bystry, R. S., Aluvihare, V., Welch, K. A., Kallikourdis, M. & Betz, A. G. (2001) Nat. Immunol. 2, 1126–1132. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

