Role of retinoid signaling in the regulation of spermatogenesis
- PMID: 15237207
- PMCID: PMC3803148
- DOI: 10.1159/000078189
Role of retinoid signaling in the regulation of spermatogenesis
Abstract
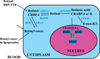
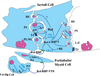
While the need for vitamin A for the normal progression of male germ cell differentiation has been known for many years, the molecular mechanisms underlying this requirement are poorly understood. This review will explore the aspects of the effects on spermatogenesis of dietary deprivation of vitamin A, in particular as to how they compare to the male sterility that results from the genetic ablation of function of the retinoid receptor RARalpha. The effects of other genes involved with retinoid synthesis, transport, and degradation are also considered. The possible cellular mechanisms that may be affected by the lack of retinoid signaling are discussed, in particular, cell cycle regulation and cell-cell interaction, both of which are critical for normal spermatogenesis.
Copyright 2004 S. Karger AG, Basel
Figures
References
-
- Abu-Abed S, MacLean G, Fraulob V, Chambon P, Petkovich M, Dolle P. Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26B1 during murine organogenesis. Mech Dev. 2002;110:173–177. - PubMed
-
- Abu-Abed S, Dolle P, Metzger D, Wood C, MacLean G, Chambon P, Petkovich M. Developing with lethal RA levels genetic ablation of Rarg can restore the viability of mice lacking Cyp26a1 . Development. 2003;130:1449–1459. - PubMed
-
- Akmal KM, Dufour JM, Kim KH. Retinoic acid receptor alpha gene expression in the rat testis: potential role during the prophase of meiosis and in the transition from round to elongating spermatids. Biol Reprod. 1997;56:549–556. - PubMed
-
- Altucci L, Gronemeyer H. Nuclear receptors in cell life and death. Trends Endocrinol Metab. 2001;12:460–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

