A small aptamer with strong and specific recognition of the triphosphate of ATP
- PMID: 15237981
- PMCID: PMC4983724
- DOI: 10.1021/ja049171k
A small aptamer with strong and specific recognition of the triphosphate of ATP
Abstract
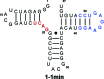
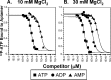
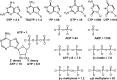
We report the in vitro selection of an RNA-based ATP aptamer with the ability to discriminate between adenosine ligands based on their 5' phosphorylation state. Previous selection of ATP aptamers yielded molecules that do not significantly discriminate between ligands at the 5' position. By applying a selective pressure that demands recognition of the 5' triphosphate, we obtained an aptamer that binds to ATP with a Kd of approximately 5 muM, and to AMP with a Kd of approximately 5.5 mM, a difference of 1100-fold. This aptamer demonstrates the ability of small RNAs to interact with negatively charged moieties.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

