The NADPH oxidase of professional phagocytes--prototype of the NOX electron transport chain systems
- PMID: 15238208
- PMCID: PMC2636547
- DOI: 10.1016/j.bbabio.2004.03.008
The NADPH oxidase of professional phagocytes--prototype of the NOX electron transport chain systems
Abstract
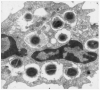
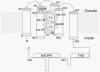
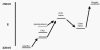
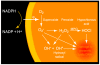
The NADPH oxidase is an electron transport chain in "professional" phagocytic cells that transfers electrons from NADPH in the cytoplasm, across the wall of the phagocytic vacuole, to form superoxide. The electron transporting flavocytochrome b is activated by the integrated function of four cytoplasmic proteins. The antimicrobial function of this system involves pumping K+ into the vacuole through BKCa channels, the effect of which is to elevate the vacuolar pH and activate neutral proteases. A number of homologous systems have been discovered in plants and lower animals as well as in man. Their function remains to be established.
Figures
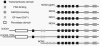





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

