In vivo folding of recombinant metallo-beta-lactamase L1 requires the presence of Zn(II)
- PMID: 15238636
- PMCID: PMC2279831
- DOI: 10.1110/ps.04742704
In vivo folding of recombinant metallo-beta-lactamase L1 requires the presence of Zn(II)
Abstract
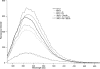
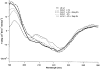
Metallo-beta-lactamase L1, secreted by pathogenic Stenotrophomonas maltophilia, is a dinuclear Zn(II)-containing enzyme that hydrolyzes almost all known penicillins, cephalosporins, and carbapenems. The presence of Zn(II) ions in both metal binding sites is essential for full enzymatic activity; however, the mechanism of physiological metal incorporation is unknown. To probe metal incorporation, L1 was over-expressed in minimal media with (mmL1+Zn) and without (mmL1-Zn) Zn(II) added to the media, and the resulting proteins were purified and characterized. The mmL1+Zn sample was bound by a Q-Sepharose column, exhibited steady-state kinetic properties, bound Zn(II), existed as a tetramer, and yielded fluorescence emission and CD spectra similar to L1 overexpressed in rich media. On the other hand, the mmL1-Zn sample did not bind to a Q-Sepharose column, and gel filtration studies demonstrated that this protein was monomeric. The mmL1-Zn sample exhibited a lower kcat value, bound less Zn(II), and yielded fluorescence emission and CD spectra consistent with this enzyme being folded improperly. Taken together, these data demonstrate that the proper folding of L1 requires the presence of Zn(II) and suggest that in vitro, thermodynamic metal binding studies do not accurately reflect physiological metal incorporation into L1.
Figures
Similar articles
-
Site-selective binding of Zn(II) to metallo-beta-lactamase L1 from Stenotrophomonas maltophilia.J Biol Inorg Chem. 2006 Apr;11(3):351-8. doi: 10.1007/s00775-006-0083-z. Epub 2006 Feb 18. J Biol Inorg Chem. 2006. PMID: 16489411
-
Metal binding Asp-120 in metallo-beta-lactamase L1 from Stenotrophomonas maltophilia plays a crucial role in catalysis.J Biol Chem. 2004 Jan 9;279(2):920-7. doi: 10.1074/jbc.M309852200. Epub 2003 Oct 22. J Biol Chem. 2004. PMID: 14573595
-
Structural and biochemical analysis of the metallo-β-lactamase L1 from emerging pathogen Stenotrophomonas maltophilia revealed the subtle but distinct di-metal scaffold for catalytic activity.Protein Sci. 2020 Mar;29(3):723-743. doi: 10.1002/pro.3804. Epub 2019 Dec 24. Protein Sci. 2020. PMID: 31846104 Free PMC article.
-
Probing substrate binding to metallo-beta-lactamase L1 from Stenotrophomonas maltophilia by using site-directed mutagenesis.BMC Biochem. 2002;3:4. doi: 10.1186/1471-2091-3-4. Epub 2002 Feb 13. BMC Biochem. 2002. PMID: 11876827 Free PMC article.
-
Metallo-beta-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily.Biochem Pharmacol. 2007 Dec 15;74(12):1686-701. doi: 10.1016/j.bcp.2007.05.021. Epub 2007 Jun 2. Biochem Pharmacol. 2007. PMID: 17597585 Review.
Cited by
-
The quorum-quenching metallo-gamma-lactonase from Bacillus thuringiensis exhibits a leaving group thio effect.Biochemistry. 2006 Nov 7;45(44):13385-93. doi: 10.1021/bi061238o. Biochemistry. 2006. PMID: 17073460 Free PMC article.
-
Membrane anchoring stabilizes and favors secretion of New Delhi metallo-β-lactamase.Nat Chem Biol. 2016 Jul;12(7):516-22. doi: 10.1038/nchembio.2083. Epub 2016 May 16. Nat Chem Biol. 2016. PMID: 27182662 Free PMC article.
-
Structure and mechanism of copper- and nickel-substituted analogues of metallo-beta-lactamase L1.Biochemistry. 2009 Apr 7;48(13):2981-9. doi: 10.1021/bi802295z. Biochemistry. 2009. PMID: 19228020 Free PMC article.
-
Site-selective binding of Zn(II) to metallo-beta-lactamase L1 from Stenotrophomonas maltophilia.J Biol Inorg Chem. 2006 Apr;11(3):351-8. doi: 10.1007/s00775-006-0083-z. Epub 2006 Feb 18. J Biol Inorg Chem. 2006. PMID: 16489411
-
Motion of the zinc ions in catalysis by a dizinc metallo-beta-lactamase.J Am Chem Soc. 2009 Aug 26;131(33):11642-3. doi: 10.1021/ja902534b. J Am Chem Soc. 2009. PMID: 19653676 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

