Transient kinetics of the reaction catalysed by magnesium protoporphyrin IX methyltransferase
- PMID: 15239672
- PMCID: PMC1133978
- DOI: 10.1042/BJ20040661
Transient kinetics of the reaction catalysed by magnesium protoporphyrin IX methyltransferase
Abstract
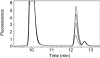
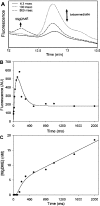
Magnesium protoporphyrin IX methyltransferase (ChlM), an enzyme in the chlorophyll biosynthetic pathway, catalyses the transfer of a methyl group to magnesium protoporphyrin IX (MgP) to form magnesium protoporphyrin IX monomethyl ester (MgPME). S-Adenosyl-L-methionine is the other substrate, from which a methyl group is transferred to the propionate group on ring C of the porphyrin macrocycle. Stopped-flow techniques were used to characterize the binding of porphyrin substrate to ChlM from Synechocystis PCC6803 by monitoring tryptophan fluorescence quenching on a millisecond timescale. We concluded that a rapid binding step is preceded by a slower isomerization of the enzyme. Quenched-flow techniques have been employed to characterize subsequent partial reactions in the catalytic mechanism. A lag phase has been identified that has been attributed to the formation of an intermediate. Our results provide a greater understanding of this catalytic process which controls the relative concentrations of MgP and MgPME, both of which are implicated in signalling between the plastid and nucleus in plants.
Figures

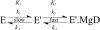

Similar articles
-
Purification and kinetic characterization of the magnesium protoporphyrin IX methyltransferase from Synechocystis PCC6803.Biochem J. 2003 Apr 15;371(Pt 2):351-60. doi: 10.1042/BJ20021394. Biochem J. 2003. PMID: 12489983 Free PMC article.
-
An enzyme-coupled continuous spectrophotometric assay for magnesium protoporphyrin IX methyltransferases.Anal Biochem. 2009 Nov 15;394(2):223-8. doi: 10.1016/j.ab.2009.07.036. Epub 2009 Jul 29. Anal Biochem. 2009. PMID: 19646414
-
Anaerobic protoporphyrin biosynthesis does not require incorporation of methyl groups from methionine.J Bacteriol. 1995 Oct;177(20):5778-83. doi: 10.1128/jb.177.20.5778-5783.1995. J Bacteriol. 1995. PMID: 7592323 Free PMC article.
-
Biosynthesis of chlorophylls from protoporphyrin IX.Nat Prod Rep. 2003 Jun;20(3):327-41. doi: 10.1039/b110549n. Nat Prod Rep. 2003. PMID: 12828371 Review.
-
Mechanisms of regulation and interplastid localization of chlorophyll biosynthesis.Membr Cell Biol. 1998;12(5):627-43. Membr Cell Biol. 1998. PMID: 10379645 Review.
Cited by
-
Posttranslational influence of NADPH-dependent thioredoxin reductase C on enzymes in tetrapyrrole synthesis.Plant Physiol. 2013 May;162(1):63-73. doi: 10.1104/pp.113.217141. Epub 2013 Apr 8. Plant Physiol. 2013. PMID: 23569108 Free PMC article.
-
Biosynthesis of the modified tetrapyrroles-the pigments of life.J Biol Chem. 2020 May 15;295(20):6888-6925. doi: 10.1074/jbc.REV120.006194. Epub 2020 Apr 2. J Biol Chem. 2020. PMID: 32241908 Free PMC article. Review.
-
S-adenosyl-L-methionine:magnesium-protoporphyrin IX O-methyltransferase from Rhodobacter capsulatus: mechanistic insights and stimulation with phospholipids.Biochem J. 2007 Sep 15;406(3):469-78. doi: 10.1042/BJ20070284. Biochem J. 2007. PMID: 17594291 Free PMC article.
-
Impaired Magnesium Protoporphyrin IX Methyltransferase (ChlM) Impedes Chlorophyll Synthesis and Plant Growth in Rice.Front Plant Sci. 2017 Sep 28;8:1694. doi: 10.3389/fpls.2017.01694. eCollection 2017. Front Plant Sci. 2017. PMID: 29033966 Free PMC article.
-
Recent advances in chlorophyll biosynthesis.Photosynth Res. 2006 Nov;90(2):173-94. doi: 10.1007/s11120-006-9076-6. Photosynth Res. 2006. PMID: 17370354 Review.
References
-
- Heyes D. J., Martin G. E., Reid R. J., Hunter C. N., Wilks H. M. NADPH:protochlorophyllide oxidoreductase from Synechocystis: overexpression, purification and preliminary characterisation. FEBS Lett. 2000;483:47–51. - PubMed
-
- Oster U., Bauer C. E., Rüdiger W. Characterization of chlorophyll a and bacteriochlorophyll a synthases by heterologous expression in Escherichia coli. J. Biol. Chem. 1997;272:9671–9676. - PubMed
-
- Keller Y., Bouvier F., d'Harlingue A., Camara B. Metabolic compartmentation of plastid prenyllipid biosynthesis – evidence for the involvement of a multifunctional geranylgeranyl reductase. Eur. J. Biochem. 1998;251:413–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

