Development of a multispecies oral bacterial community in a saliva-conditioned flow cell
- PMID: 15240317
- PMCID: PMC444820
- DOI: 10.1128/AEM.70.7.4340-4348.2004
Development of a multispecies oral bacterial community in a saliva-conditioned flow cell
Abstract
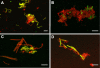
Microbial communities within the human oral cavity are dynamic associations of more than 500 bacterial species that form biofilms on the soft and hard tissues of the mouth. Understanding the development and spatial organization of oral biofilms has been facilitated by the use of in vitro models. We used a saliva-conditioned flow cell, with saliva as the sole nutritional source, as a model to examine the development of multispecies biofilm communities from an inoculum containing the coaggregation partners Streptococcus gordonii, Actinomyces naeslundii, Veillonella atypica, and Fusobacterium nucleatum. Biofilms inoculated with individual species in a sequential order were compared with biofilms inoculated with coaggregates of the four species. Our results indicated that flow cells inoculated sequentially produced biofilms with larger biovolumes compared to those biofilms inoculated with coaggregates. Individual-species biovolumes within the four-species communities also differed between the two modes of inoculation. Fluorescence in situ hybridization with genus- and species-specific probes revealed that the majority of cells in both sequentially and coaggregate-inoculated biofilms were S. gordonii, regardless of the inoculation order. However, the representation of A. naeslundii and V. atypica was significantly higher in biofilms inoculated with coaggregates compared to sequentially inoculated biofilms. Thus, these results indicate that the development of multispecies biofilm communities is influenced by coaggregations preformed in planktonic phase. Coaggregating bacteria such as certain streptococci are especially adapted to primary colonization of saliva-conditioned surfaces independent of the mode of inoculation and order of addition in the multispecies inoculum. Preformed coaggregations favor other bacterial strains and may facilitate symbiotic relationships.
Figures

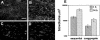




Similar articles
-
Interactions between salivary Bifidobacterium adolescentis and other oral bacteria: in vitro coaggregation and coadhesion assays.FEMS Microbiol Lett. 2008 Apr;281(2):183-9. doi: 10.1111/j.1574-6968.2008.01092.x. Epub 2008 Feb 27. FEMS Microbiol Lett. 2008. PMID: 18312575
-
Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition.Proc Natl Acad Sci U S A. 2004 Nov 30;101(48):16917-22. doi: 10.1073/pnas.0407457101. Epub 2004 Nov 16. Proc Natl Acad Sci U S A. 2004. PMID: 15546975 Free PMC article.
-
Characterization and application of a flow system for in vitro multispecies oral biofilm formation.J Periodontal Res. 2014 Jun;49(3):323-32. doi: 10.1111/jre.12110. Epub 2013 Jul 1. J Periodontal Res. 2014. PMID: 23815431
-
Oral microbial communities: biofilms, interactions, and genetic systems.Annu Rev Microbiol. 2000;54:413-37. doi: 10.1146/annurev.micro.54.1.413. Annu Rev Microbiol. 2000. PMID: 11018133 Review.
-
Microbial communities and their interactions in biofilm systems: an overview.Water Sci Technol. 2004;49(11-12):327-36. Water Sci Technol. 2004. PMID: 15303758 Review.
Cited by
-
Relationship between sucrose concentration and bacteria proportion in a multispecies biofilm: Short title: Sucrose challenges to a multispecies biofilm.J Oral Microbiol. 2021 Apr 7;13(1):1910443. doi: 10.1080/20002297.2021.1910443. J Oral Microbiol. 2021. PMID: 33889308 Free PMC article.
-
Use of a high-throughput in vitro microfluidic system to develop oral multi-species biofilms.J Vis Exp. 2014 Dec 1;(94):52467. doi: 10.3791/52467. J Vis Exp. 2014. PMID: 25490193 Free PMC article.
-
Role of Dilution Rate and Nutrient Availability in the Formation of Microbial Biofilms.Front Microbiol. 2019 Apr 30;10:916. doi: 10.3389/fmicb.2019.00916. eCollection 2019. Front Microbiol. 2019. PMID: 31114560 Free PMC article.
-
Evaluating the Effects of Disinfectants on Bacterial Biofilms Using a Microfluidics Flow Cell and Time-Lapse Fluorescence Microscopy.Microorganisms. 2020 Nov 22;8(11):1837. doi: 10.3390/microorganisms8111837. Microorganisms. 2020. PMID: 33266442 Free PMC article.
-
Experimental Models of Oral Biofilms Developed on Inert Substrates: A Review of the Literature.Biomed Res Int. 2016;2016:7461047. doi: 10.1155/2016/7461047. Epub 2016 Sep 6. Biomed Res Int. 2016. PMID: 27699173 Free PMC article. Review.
References
-
- Bos, R., H. C. van der Mei, and H. J. Busscher. 1996. Co-adhesion of oral microbial pairs under flow in the presence of saliva and lactose. J. Dent. Res. 75:809-815. - PubMed
-
- Bos, R., H. C. van der Mei, J. M. Meinders, and H. J. Busscher. 1994. A quantitative method to study co-adhesion of microorganisms in a parellel plate flow chamber: basic principles of the analysis. J. Microbiol. Meth. 20:289-305.
-
- Bradshaw, D. J., P. D. Marsh, C. Allison, and K. M. Schilling. 1996. Effect of oxygen, inoculum composition and flow rate on development of mixed-culture oral biofilms. Microbiology 142:623-629. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

