A new method for the reconstitution of membrane proteins into giant unilamellar vesicles
- PMID: 15240476
- PMCID: PMC1304363
- DOI: 10.1529/biophysj.104.040360
A new method for the reconstitution of membrane proteins into giant unilamellar vesicles
Erratum in
- Biophys J. 2004 Sep;87(3):2098
Abstract
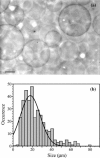
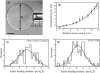
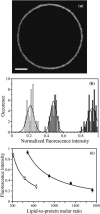
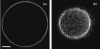
In this work, we have investigated a new and general method for the reconstitution of membrane proteins into giant unilamellar vesicles (GUVs). We have analyzed systematically the reconstitution of two radically different membrane proteins, the sarcoplasmic reticulum Ca(2+)-ATPase and the H(+) pump bacteriorhodopsin. In a first step, our method involved a detergent-mediated reconstitution of solubilized membrane proteins into proteoliposomes of 0.1-0.2 microm in size. In a second step, these preformed proteoliposomes were partially dried under controlled humidity followed, in a third step, by electroswelling of the partially dried film to give GUVs. The physical characteristics of GUVs were analyzed in terms of morphology, size, and lamellarity using phase-contrast and differential interference contrast microscopy. The reconstitution process was further characterized by analyzing protein incorporation and biological activity. Both membrane proteins could be homogeneously incorporated into GUVs at lipid/protein ratios ranging from 5 to 40 (w/w). After reconstitution, both proteins retained their biological activity as demonstrated by H(+) or Ca(2+) pumping driven by bacteriorhodopsin or Ca(2+)-ATPase, respectively. This constitutes an efficient new method of reconstitution, leading to the production of large unilamellar membrane protein-containing vesicles of more than 20 microm in diameter, which should prove useful for functional and structural studies through the use of optical microscopy, optical tweezers, microelectrodes, or atomic force microscopy.
Figures


References
-
- Abdelghani-Jacquin, C., A. Abdelghani, M. Kantlehner, and E. Sackmann. 2002. Decorated surfaces by biofunctionalized gold beads: application to cell adhesion studies. Eur. Biophys. J. 2:102–110. - PubMed
-
- Ajouz, B., C. Berrier, M. Besnard, B. Martinac, and A. Ghazi. 2000. Contributions of the different extramembranous domains of the mechanosensitive ion channel MscL to its response to membrane tension. J. Biol. Chem. 275:1015–1022. - PubMed
-
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. D. Watson. 1994. Molecular Biology of the Cell, 3rd ed. Garland Publishing, New York.
-
- Angelova, M. I. 2000. Liposome electroformation. In Giant Vesicles. P. L. Luisi and P. Walde, editors. John Wiley and Sons, Chichester, UK. 27–36.
-
- Angelova, M. I., N. Hristova, and I. Tsoneva. 1999. DNA-induced endocytosis upon local microinjection to giant unilamellar cationic vesicles. Eur. Biophys. J. 28:142–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

