A nanosensor for transmembrane capture and identification of single nucleic Acid molecules
- PMID: 15240494
- PMCID: PMC1304383
- DOI: 10.1529/biophysj.104.040212
A nanosensor for transmembrane capture and identification of single nucleic Acid molecules
Erratum in
- Biophys J. 2004 Nov;87(5):3618
Abstract
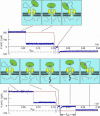
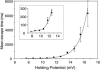
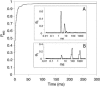
We have engineered a nanosensor for sequence-specific detection of single nucleic acid molecules across a lipid bilayer. The sensor is composed of a protein channel nanopore (alpha-hemolysin) housing a DNA probe with an avidin anchor at the 5' end and a nucleotide sequence designed to noncovalently bind a specific single-stranded oligonucleotide at the 3' end. The 3' end of the DNA probe is driven to the opposite side of the pore by an applied electric potential, where it can specifically bind to oligonucleotides. Reversal of the applied potential withdraws the probe from the pore, dissociating it from a bound oligonucleotide. The time required for dissociation of the probe-oligonucleotide duplex under this force yields identifying characteristics of the oligonucleotide. We demonstrate transmembrane detection of individual oligonucleotides, discriminate between molecules differing by a single nucleotide, and investigate the relationship between dissociation time and hybridization energy of the probe and analyte molecules. The detection method presented in this article is a candidate for in vivo single-molecule detection and, through parallelization in a synthetic device, for genotyping and global transcription profiling from small samples.
Figures



References
-
- Evans, E. 2001. Probing the relation between force lifetime and chemistry in single molecular bonds. Annu. Rev. Biophys. Biomol. Struct. 30:105–128. - PubMed
-
- Henrickson, S. E., M. Misakian, B. Robertson, and J. J. Kasianowicz. 2000. Driven DNA transport into an asymmetric nanometer-scale pore. Phys. Rev. Lett. 85:3057–3060. - PubMed
-
- Howorka, S., S. Cheley, and H. Bayley. 2001a. Sequence-specific detection of individual DNA strands using engineered nanopores. Nat. Biotechnol. 19:636–639. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

