Novel spectroscopic technique for in situ monitoring of collagen fibril alignment in gels
- PMID: 15240498
- PMCID: PMC1304387
- DOI: 10.1529/biophysj.103.038976
Novel spectroscopic technique for in situ monitoring of collagen fibril alignment in gels
Abstract
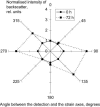
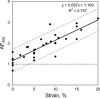
Development of collagen fibril alignment in contracting fibroblast-populated and externally tensioned acellular collagen gels was studied using elastic scattering spectroscopy. Spectra of the backscattered light (320-860 nm) were acquired with a 2.75-mm source-detector separation probe placed perpendicular to the gel surface and rotated to achieve different angles to the collagen fibril alignment. Backscatter was isotropic for noncontracted/unloaded gels (disorganized matrix). As gels were contracted/externally loaded (collagen alignment developed), anisotropy of backscatter increased: more backscatter was detected perpendicular than parallel to the direction of the fibril alignment. An "anisotropy factor" (AF) was calculated to characterize this effect as the ratio of backscatter intensities at orthogonal positions. Before contraction (or zero strain) the AF was close to unity at all wavelengths. In contrast, at 72 h, backscatter anisotropy varied from AF(400 nm) = 2.14 +/- 0.29 to AF(700 nm) = 3.04 +/- 0.48. It also increased over threefold up to a strain of 20%. The AF strongly correlated with the contraction time/strain. Different directions of the backscatter were detected in gel zones with known differences in the matrix alignment. Thus, backscatter anisotropy allows in situ nondestructive determination of collagen fibril alignment and quantitative monitoring of its development.
Figures







Similar articles
-
In situ monitoring of tendon structural changes by elastic scattering spectroscopy: correlation with changes in collagen fibril diameter and crimp.Tissue Eng. 2006 Jul;12(7):1821-31. doi: 10.1089/ten.2006.12.1821. Tissue Eng. 2006. PMID: 16889512
-
Structural changes in loaded equine tendons can be monitored by a novel spectroscopic technique.J Physiol. 2004 Feb 1;554(Pt 3):791-801. doi: 10.1113/jphysiol.2003.054809. Epub 2003 Oct 24. J Physiol. 2004. PMID: 14578479 Free PMC article.
-
In the beginning there were soft collagen-cell gels: towards better 3D connective tissue models?Exp Cell Res. 2013 Oct 1;319(16):2460-9. doi: 10.1016/j.yexcr.2013.07.001. Epub 2013 Jul 12. Exp Cell Res. 2013. PMID: 23856376 Review.
-
Canine ACL fibroblast integrin expression and cell alignment in response to cyclic tensile strain in three-dimensional collagen gels.J Orthop Res. 2006 Mar;24(3):481-90. doi: 10.1002/jor.20050. J Orthop Res. 2006. PMID: 16453340
-
Collagen fiber alignment does not explain mechanical anisotropy in fibroblast populated collagen gels.J Biomech Eng. 2007 Oct;129(5):642-50. doi: 10.1115/1.2768104. J Biomech Eng. 2007. PMID: 17887889
Cited by
-
Hierarchical Structure and Properties of the Bone at Nano Level.Bioengineering (Basel). 2022 Nov 10;9(11):677. doi: 10.3390/bioengineering9110677. Bioengineering (Basel). 2022. PMID: 36354587 Free PMC article.
-
Characterization of engineered tissue development under biaxial stretch using nonlinear optical microscopy.Tissue Eng Part A. 2009 Jul;15(7):1553-64. doi: 10.1089/ten.tea.2008.0287. Tissue Eng Part A. 2009. PMID: 19063662 Free PMC article.
-
Techniques to assess bone ultrastructure organization: orientation and arrangement of mineralized collagen fibrils.J R Soc Interface. 2016 Jun;13(119):20160088. doi: 10.1098/rsif.2016.0088. J R Soc Interface. 2016. PMID: 27335222 Free PMC article. Review.
-
External mechanical loading overrules cell-cell mechanical communication in sprouting angiogenesis during early bone regeneration.PLoS Comput Biol. 2023 Nov 13;19(11):e1011647. doi: 10.1371/journal.pcbi.1011647. eCollection 2023 Nov. PLoS Comput Biol. 2023. PMID: 37956208 Free PMC article.
-
Quantification of the temporal evolution of collagen orientation in mechanically conditioned engineered cardiovascular tissues.Ann Biomed Eng. 2009 Jul;37(7):1263-72. doi: 10.1007/s10439-009-9698-x. Epub 2009 May 5. Ann Biomed Eng. 2009. PMID: 19415496 Free PMC article.
References
-
- Barocas V. H., A. G. Moon, and R. T. Tranquillo. 1995. The fibroblast-populated collagen microsphere assay of cell traction force. Part 2. Measurement of the cell traction parameter. J Biomech. Engr. 117: 161–170. - PubMed
-
- Bigio, I. J., and J. R. Mourant. 1997. Ultraviolet and visible spectroscopies for tissue diagnostics: fluorescence spectroscopy and elastic-scattering spectroscopy. Phys. Med. Biol. 42:803–814. - PubMed
-
- Bohren, C. F., and D. R. Huffman. 1983. Absorption and scattering of light by small particles. Wiley, New York.
-
- Brown, R. A., R. Prajapati, D. A. McGrouther, I. V. Yannas, and M. Eastwood. 1998. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J. Cell. Physiol. 175:323–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

