Analysis of functional coupling: mitochondrial creatine kinase and adenine nucleotide translocase
- PMID: 15240503
- PMCID: PMC1304393
- DOI: 10.1529/biophysj.103.036210
Analysis of functional coupling: mitochondrial creatine kinase and adenine nucleotide translocase
Abstract
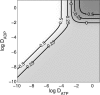
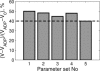
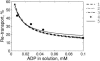
The mechanism of functional coupling between mitochondrial creatine kinase (MiCK) and adenine nucleotide translocase (ANT) in isolated heart mitochondria is analyzed. Two alternative mechanisms are studied: 1), dynamic compartmentation of ATP and ADP, which assumes the differences in concentrations of the substrates between intermembrane space and surrounding solution due to some diffusion restriction and 2), direct transfer of the substrates between MiCK and ANT. The mathematical models based on these possible mechanisms were composed and simulation results were compared with the available experimental data. The first model, based on a dynamic compartmentation mechanism, was not sufficient to reproduce the measured values of apparent dissociation constants of MiCK reaction coupled to oxidative phosphorylation. The second model, which assumes the direct transfer of substrates between MiCK and ANT, is shown to be in good agreement with experiments--i.e., the second model reproduced the measured constants and the estimated ADP flux, entering mitochondria after the MiCK reaction. This model is thermodynamically consistent, utilizing the free energy profiles of reactions. The analysis revealed the minimal changes in the free energy profile of the MiCK-ANT interaction required to reproduce the experimental data. A possible free energy profile of the coupled MiCK-ANT system is presented.
Figures







Similar articles
-
Quantitative analysis of the 'phosphocreatine shuttle': I. A probability approach to the description of phosphocreatine production in the coupled creatine kinase-ATP/ADP translocase-oxidative phosphorylation reactions in heart mitochondria.Biochim Biophys Acta. 1993 Jul 26;1143(3):291-300. doi: 10.1016/0005-2728(93)90200-y. Biochim Biophys Acta. 1993. PMID: 8329438
-
Theoretical modelling of some spatial and temporal aspects of the mitochondrion/creatine kinase/myofibril system in muscle.Mol Cell Biochem. 1998 Jul;184(1-2):249-89. Mol Cell Biochem. 1998. PMID: 9746325
-
Regulation of respiration in brain mitochondria and synaptosomes: restrictions of ADP diffusion in situ, roles of tubulin, and mitochondrial creatine kinase.Mol Cell Biochem. 2008 Nov;318(1-2):147-65. doi: 10.1007/s11010-008-9865-7. Epub 2008 Jul 16. Mol Cell Biochem. 2008. PMID: 18629616
-
Mathematical modeling of intracellular transport processes and the creatine kinase systems: a probability approach.Mol Cell Biochem. 1994 Apr-May;133-134:333-46. Mol Cell Biochem. 1994. PMID: 7808463 Review.
-
The creatine kinase phosphotransfer network: thermodynamic and kinetic considerations, the impact of the mitochondrial outer membrane and modelling approaches.Subcell Biochem. 2007;46:27-65. doi: 10.1007/978-1-4020-6486-9_3. Subcell Biochem. 2007. PMID: 18652071 Review.
Cited by
-
Mitochondria in cardiomyocyte Ca2+ signaling.Int J Biochem Cell Biol. 2009 Oct;41(10):1957-71. doi: 10.1016/j.biocel.2009.03.011. Epub 2009 Apr 2. Int J Biochem Cell Biol. 2009. PMID: 19703657 Free PMC article. Review.
-
Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival.Cell Death Dis. 2015 Jul 30;6(7):e1835. doi: 10.1038/cddis.2015.202. Cell Death Dis. 2015. PMID: 26225774 Free PMC article.
-
Cardiac system bioenergetics: metabolic basis of the Frank-Starling law.J Physiol. 2006 Mar 1;571(Pt 2):253-73. doi: 10.1113/jphysiol.2005.101444. Epub 2006 Jan 12. J Physiol. 2006. PMID: 16410283 Free PMC article. Review.
-
Modeling of oxygen transport and cellular energetics explains observations on in vivo cardiac energy metabolism.PLoS Comput Biol. 2006 Sep 15;2(9):e107. doi: 10.1371/journal.pcbi.0020107. Epub 2006 Jul 10. PLoS Comput Biol. 2006. PMID: 16978045 Free PMC article.
-
Strong inference for systems biology.PLoS Comput Biol. 2009 Aug;5(8):e1000459. doi: 10.1371/journal.pcbi.1000459. Epub 2009 Aug 28. PLoS Comput Biol. 2009. PMID: 19714210 Free PMC article. No abstract available.
References
-
- Abraham, M. R., V. A. Selivanov, D. M. Hodgson, D. Pucar, L. V. Zingman, B. Wieringa, P. P. Dzeja, A. E. Alekseev, and A. Terzic. 2002. Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J. Biol. Chem. 277:24427–24434. - PubMed
-
- Aliev, M. K., and V. A. Saks. 1993. Quantitative analysis of the “phosphocreatine shuttle.” I. A probability approach to the description of phosphocreatine production in the coupled creatine kinase-ATP/ADP translocase-oxidative phosphorylation reactions. Biochim. Biophys. Acta. 1143:291–300. - PubMed
-
- Aliev, M. K., and V. A. Saks. 1994. Mathematical modeling of intracellular transport processes and the creatine kinase systems: a probability approach. Mol. Cell. Biochem. 133–134:333–346. - PubMed
-
- Barbour, R. L., J. Ribaudo, and S. H. Chan. 1984. Effect of creatine kinase activity on mitochondrial ADP/ATP transport. Evidence for a functional interaction. J. Biol. Chem. 259:8246–8251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

