Determination of cell capacitance using the exact empirical solution of partial differential Y/partial differential Cm and its phase angle
- PMID: 15240504
- PMCID: PMC1304394
- DOI: 10.1529/biophysj.103.033993
Determination of cell capacitance using the exact empirical solution of partial differential Y/partial differential Cm and its phase angle
Abstract
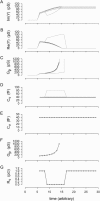
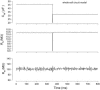
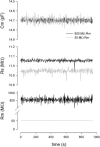
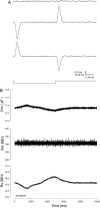
Measures of membrane capacitance offer insight into a variety of cellular processes. Unfortunately, popular methodologies rely on model simplifications that sensitize them to interference from inevitable changes in resistive components of the traditional cell-clamp model. Here I report on a novel method to measure membrane capacitance that disposes of the usual simplifications and assumptions, yet is immune to such interference and works on the millisecond timescale. It is based on the exact empirical determination of the elusive partial derivative, partial differential Y/partial differential C(m), which heretofore had been approximated. Furthermore, I illustrate how this method extends to the vesicle fusion problem by permitting the determination of partial differential Y(v)/partial differential C(v), thereby providing estimates of fusion pore conductance and vesicle capacitance. Finally, I provide simulation examples and physiological examples of how the method can be used to study processes that are routinely interrogated by measures of membrane capacitance.
Figures



Similar articles
-
Determination of Cell Membrane Capacitance and Conductance via Optically Induced Electrokinetics.Biophys J. 2017 Oct 3;113(7):1531-1539. doi: 10.1016/j.bpj.2017.08.006. Biophys J. 2017. PMID: 28978446 Free PMC article.
-
Influence of conductance changes on patch clamp capacitance measurements using a lock-in amplifier and limitations of the phase tracking technique.Biophys J. 1995 Dec;69(6):2808-22. doi: 10.1016/S0006-3495(95)80154-2. Biophys J. 1995. PMID: 8599687 Free PMC article.
-
Fusion pore conductance: experimental approaches and theoretical algorithms.Biophys J. 1998 May;74(5):2374-87. doi: 10.1016/S0006-3495(98)77946-9. Biophys J. 1998. PMID: 9591664 Free PMC article.
-
Error analysis of Cm measurement under the whole-cell patch-clamp recording.J Neurosci Methods. 2010 Jan 15;185(2):307-14. doi: 10.1016/j.jneumeth.2009.10.003. Epub 2009 Oct 14. J Neurosci Methods. 2010. PMID: 19835911
-
Computer Simulation of Partial Discharges in Voids inside Epoxy Resins Using Three-Capacitance and Analytical Models.Polymers (Basel). 2020 Jan 2;12(1):77. doi: 10.3390/polym12010077. Polymers (Basel). 2020. PMID: 31906561 Free PMC article. Review.
Cited by
-
Zf-and-Hsys-based Cm measurement under the whole-cell patch-clamp recording.Pflugers Arch. 2009 Apr;457(6):1423-34. doi: 10.1007/s00424-008-0614-2. Epub 2008 Dec 2. Pflugers Arch. 2009. PMID: 19048285
-
N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein.Biophys J. 2005 Nov;89(5):3345-52. doi: 10.1529/biophysj.105.068759. Epub 2005 Aug 19. Biophys J. 2005. PMID: 16113116 Free PMC article.
-
On membrane motor activity and chloride flux in the outer hair cell: lessons learned from the environmental toxin tributyltin.Biophys J. 2005 Mar;88(3):2350-62. doi: 10.1529/biophysj.104.053579. Epub 2004 Dec 13. Biophys J. 2005. PMID: 15596517 Free PMC article.
-
Maturation of Voltage-induced Shifts in SLC26a5 (Prestin) Operating Point during Trafficking and Membrane Insertion.Neuroscience. 2020 Apr 1;431:128-133. doi: 10.1016/j.neuroscience.2020.02.003. Epub 2020 Feb 13. Neuroscience. 2020. PMID: 32061780 Free PMC article.
-
Thyroid hormone is required for pruning, functioning and long-term maintenance of afferent inner hair cell synapses.Eur J Neurosci. 2016 Jan;43(2):148-61. doi: 10.1111/ejn.13081. Epub 2015 Oct 28. Eur J Neurosci. 2016. PMID: 26386265 Free PMC article.
References
-
- Ales, E., L. Tabares, J. M. Poyato, V. Valero, M. Lindau, and D. T. Alvarez. 1999. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat. Cell Biol. 1:40–44. - PubMed
-
- Alvarez De Toledo, G. A., R. Fernandez-Chacon, and J. M. Fernandez. 1993. Release of secretory products during transient vesicle fusion. Nature. 363:554–558. - PubMed
-
- Aravanis, A. M., J. L. Pyle, and R. W. Tsien. 2003. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 423:643–647. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

