SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes
- PMID: 15240568
- PMCID: PMC2172132
- DOI: 10.1083/jcb.200311021
SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes
Abstract
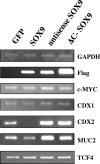
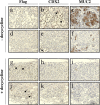
TCF and SOX proteins belong to the high mobility group box transcription factor family. Whereas TCFs, the transcriptional effectors of the Wnt pathway, have been widely implicated in the development, homeostasis and disease of the intestine epithelium, little is known about the function of the SOX proteins in this tissue. Here, we identified SOX9 in a SOX expression screening in the mouse fetal intestine. We report that the SOX9 protein is expressed in the intestinal epithelium in a pattern characteristic of Wnt targets. We provide in vitro and in vivo evidence that a bipartite beta-catenin/TCF4 transcription factor, the effector of the Wnt signaling pathway, is required for SOX9 expression in epithelial cells. Finally, in colon epithelium-derived cells, SOX9 transcriptionally represses the CDX2 and MUC2 genes, normally expressed in the mature villus cells of the intestinal epithelium, and may therefore contribute to the Wnt-dependent maintenance of a progenitor cell phenotype.
Figures
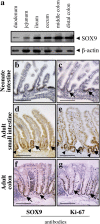
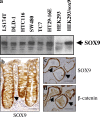
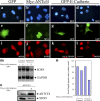
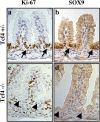
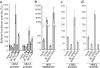
References
-
- Aoki, K., Y. Tamai, S. Horiike, M. Oshima, and M.M. Taketo. 2003. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Δ716 Cdx2+/− compound mutant mice. Nat. Genet. 35:323–330. - PubMed
-
- Batlle, E., J.T. Henderson, H. Beghtel, M.M.W. van den Born, E. Sancho, G. Huls, J. Meeldijk, J. Robertson, M. van de Wetering, T. Pawson, and H. Clevers. 2002. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/EphrinB. Cell. 111:251–263. - PubMed
-
- Bell, D.M., K.K.H. Leung, S.C. Wheatley, L.J. Ng, S. Zhou, K.W. Ling, M.H. Sham, P. Koopman, P.P.L. Tam, and K.S.E. Cheah. 1997. SOX9 directly regulates the type-II collagen gene. Nat. Genet. 16:174–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

