Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice
- PMID: 15240800
- PMCID: PMC6729668
- DOI: 10.1523/JNEUROSCI.5635-03.2004
Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice
Abstract
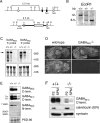
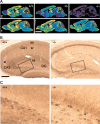
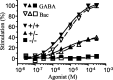
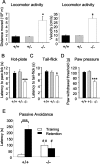
GABAB receptors mediate slow synaptic inhibition in the nervous system. In transfected cells, functional GABAB receptors are usually only observed after coexpression of GABAB(1) and GABAB(2) subunits, which established the concept of heteromerization for G-protein-coupled receptors. In the heteromeric receptor, GABAB(1) is responsible for binding of GABA, whereas GABAB(2) is necessary for surface trafficking and G-protein coupling. Consistent with these in vitro observations, the GABAB(1) subunit is also essential for all GABAB signaling in vivo. Mice lacking the GABAB(1) subunit do not exhibit detectable electrophysiological, biochemical, or behavioral responses to GABAB agonists. However, GABAB(1) exhibits a broader cellular expression pattern than GABAB(2), suggesting that GABAB(1) could be functional in the absence of GABAB(2). We now generated GABAB(2)-deficient mice to analyze whether GABAB(1) has the potential to signal without GABAB(2) in neurons. We show that GABAB(2)-/- mice suffer from spontaneous seizures, hyperalgesia, hyperlocomotor activity, and severe memory impairment, analogous to GABAB(1)-/- mice. This clearly demonstrates that the lack of heteromeric GABAB(1,2) receptors underlies these phenotypes. To our surprise and in contrast to GABAB(1)-/- mice, we still detect atypical electrophysiological GABAB responses in hippocampal slices of GABAB(2)-/- mice. Furthermore, in the absence of GABAB(2), the GABAB(1) protein relocates from distal neuronal sites to the soma and proximal dendrites. Our data suggest that association of GABAB(2) with GABAB(1) is essential for receptor localization in distal processes but is not absolutely necessary for signaling. It is therefore possible that functional GABAB receptors exist in neurons that naturally lack GABAB(2) subunits.
Figures





References
-
- Anderson WW, Collingridge GL (2001) The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods 108: 71-83. - PubMed
-
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M (2004) Molecular structure and physiological functions of GABAB receptors. Physiol Rev, in press. - PubMed
-
- Billinton A, Ige AO, Wise A, White JH, Disney GH, Marshall FH, Waldvogel HJ, Faull RL, Emson PC (2000) GABAB receptor heterodimer-component localisation in human brain. Brain Res Mol Brain Res 77: 111-124. - PubMed
-
- Bouvier M (2001) Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci 2: 274-286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
