In vivo reconstitution of the negative feedback in nitric oxide/cGMP signaling: role of phosphodiesterase type 5 phosphorylation
- PMID: 15240816
- PMCID: PMC515337
- DOI: 10.1091/mbc.e03-12-0890
In vivo reconstitution of the negative feedback in nitric oxide/cGMP signaling: role of phosphodiesterase type 5 phosphorylation
Abstract
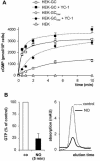
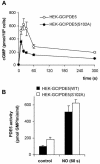
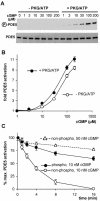
Most effects of the messenger molecule nitric oxide (NO) are mediated by cGMP, which is formed by NO-sensitive guanylyl cyclase (GC) and degraded by phosphodiesterases (PDEs). In platelets, NO elicits a spike-like cGMP response and causes a sustained desensitization. Both characteristics have been attributed to PDE5 activation caused by cGMP binding to its regulatory GAF domain. Activation is paralleled by phosphorylation whose precise function remains unknown. Here, we report reconstitution of all features of the NO-induced cGMP response in human embryonic kidney cells by coexpressing NO-sensitive GC and PDE5. The spike-like cGMP response was blunted when PDE5 phosphorylation was enhanced by additional overexpression of cGMP-dependent protein kinase. Analysis of PDE5 activation in vitro revealed a discrepancy between the cGMP concentrations required for activation (micromolar) and reversal of activation (nanomolar), indicating the conversion of a low-affinity state to a high-affinity state upon binding of cGMP. Phosphorylation even increased the high apparent affinity enabling PDE5 activation to persist at extremely low cGMP concentrations. Our data suggest that the spike-like shape and the desensitization of the cGMP response are potentially inherent to every GC- and PDE5-expressing cell. Phosphorylation of PDE5 seems to act as memory switch for activation leading to long-term desensitization of the signaling pathway.
Figures




Similar articles
-
Direct activation of PDE5 by cGMP: long-term effects within NO/cGMP signaling.J Cell Biol. 2003 Mar 3;160(5):719-27. doi: 10.1083/jcb.200211041. Epub 2003 Feb 25. J Cell Biol. 2003. PMID: 12604588 Free PMC article.
-
Inhibition of phosphodiesterase type 5 by the activator of nitric oxide-sensitive guanylyl cyclase BAY 41-2272.Circulation. 2004 Apr 13;109(14):1711-3. doi: 10.1161/01.CIR.0000126286.47618.BD. Epub 2004 Apr 5. Circulation. 2004. PMID: 15066950
-
Cyclic GMP and cGMP-binding phosphodiesterase are required for interleukin-1-induced nitric oxide synthesis in human articular chondrocytes.J Biol Chem. 1998 Oct 16;273(42):27484-91. doi: 10.1074/jbc.273.42.27484. J Biol Chem. 1998. PMID: 9765278
-
Regulation of nitric oxide-sensitive guanylyl cyclase.Circ Res. 2003 Jul 25;93(2):96-105. doi: 10.1161/01.RES.0000082524.34487.31. Circ Res. 2003. PMID: 12881475 Review.
-
Cyclic GMP phosphodiesterases and regulation of smooth muscle function.Circ Res. 2003 Aug 22;93(4):280-91. doi: 10.1161/01.RES.0000087541.15600.2B. Circ Res. 2003. PMID: 12933699 Review.
Cited by
-
Nitric oxide and cGMP protein kinase (cGK) regulate dendritic-cell migration toward the lymph-node-directing chemokine CCL19.Blood. 2006 Feb 15;107(4):1537-45. doi: 10.1182/blood-2005-07-2901. Epub 2005 Oct 25. Blood. 2006. PMID: 16249377 Free PMC article.
-
Picomolar nitric oxide signals from central neurons recorded using ultrasensitive detector cells.J Biol Chem. 2011 Dec 16;286(50):43172-81. doi: 10.1074/jbc.M111.289777. Epub 2011 Oct 20. J Biol Chem. 2011. PMID: 22016390 Free PMC article.
-
Improved genetically-encoded, FlincG-type fluorescent biosensors for neural cGMP imaging.Front Mol Neurosci. 2013 Sep 24;6:26. doi: 10.3389/fnmol.2013.00026. eCollection 2013. Front Mol Neurosci. 2013. PMID: 24068983 Free PMC article.
-
Lack of effect of ODQ does not exclude cGMP signalling via NO-sensitive guanylyl cyclase.Br J Pharmacol. 2013 Sep;170(2):317-27. doi: 10.1111/bph.12275. Br J Pharmacol. 2013. PMID: 23763290 Free PMC article.
-
A label-free LC/MS-based enzymatic activity assay for the detection of PDE5A inhibitors.Front Chem. 2023 Feb 13;11:1097027. doi: 10.3389/fchem.2023.1097027. eCollection 2023. Front Chem. 2023. PMID: 36860644 Free PMC article.
References
-
- Corbin, J.D., Blount, M.A., Weeks, J.L., 2nd, Beasley, A., Kuhn, K.P., Ho, Y.S., Saidi, L.F., Hurley, J.H., Kotera, J., and Francis, S.H. (2003). [3H]sildenafil binding to phosphodiesterase-5 is specific, kinetically heterogeneous, and stimulated by cGMP. Mol. Pharmacol. 63, 1364-1372. - PubMed
-
- Corbin, J.D., Turko, I.V., Beasley, A., and Francis, S.H. (2000). Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur. J. Biochem. 267, 2760-2767. - PubMed
-
- Francis, S.H., Bessay, E.P., Kotera, J., Grimes, K.A., Liu, L., Thompson, W.J., and Corbin, J.D. (2002). Phosphorylation of isolated human phosphodiesterase-5 regulatory domain induces an apparent conformational change and increases cGMP binding affinity. J. Biol. Chem. 277, 47581-47587. - PubMed
-
- Francis, S.H., Turko, I.V., and Corbin, J.D. (2000). Cyclic nucleotide phosphodiesterases: relating structure and function. Prog. Nucleic. Acid. Res. Mol. Biol. 65, 1-52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

