The growth-regulatory protein HCRP1/hVps37A is a subunit of mammalian ESCRT-I and mediates receptor down-regulation
- PMID: 15240819
- PMCID: PMC515363
- DOI: 10.1091/mbc.e04-03-0250
The growth-regulatory protein HCRP1/hVps37A is a subunit of mammalian ESCRT-I and mediates receptor down-regulation
Abstract
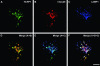
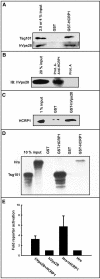
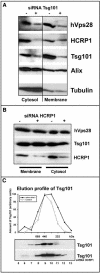
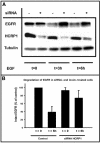
The biogenesis of multivesicular bodies and endosomal sorting of membrane cargo are driven forward by the endosomal sorting complexes required for transport, ESCRT-I, -II, and -III. ESCRT-I is characterized in yeast as a complex consisting of Vps23, Vps28, and Vps37. Whereas mammalian homologues of Vps23 and Vps28 (named Tsg101 and hVps28, respectively) have been identified and characterized, a mammalian counterpart of Vps37 has not yet been identified. Here, we show that a regulator of proliferation, hepatocellular carcinoma related protein 1 (HCRP1), interacts with Tsg101, hVps28, and their upstream regulator Hrs. The ability of HCRP1 (which we assign the alternative name hVps37A) to interact with Tsg101 is conferred by its mod(r) domain and is shared with hVps37B and hVps37C, two other mod(r) domain-containing proteins. HCRP1 cofractionates with Tsg101 and hVps28 by size exclusion chromatography and colocalizes with hVps28 on LAMP1-positive endosomes. Whereas depletion of Tsg101 by siRNA reduces cellular levels of both hVps28 and HCRP1, depletion of HCRP1 has no effect on Tsg101 or hVps28. Nevertheless, HCRP1 depletion strongly retards epidermal growth factor (EGF) receptor degradation. Together, these results indicate that HCRP1 is a subunit of mammalian ESCRT-I and that its function is essential for lysosomal sorting of EGF receptors.
Figures



References
-
- Babst, M., Katzmann, D.J., Estepa-Sabal, E.J., Meerloo, T., and Emr, S.D. (2002a). Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3, 271–282. - PubMed
-
- Babst, M., Katzmann, D.J., Snyder, W.B., Wendland, B., and Emr, S.D. (2002b). Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3, 283–289. - PubMed
-
- Babst, M., Odorizzi, G., Estepa, E.J., and Emr, S.D. (2000). Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1, 248–258. - PubMed
-
- Begley, D., Murphy, A.M., Hiu, C., and Tsubota, S.I. (1995). Modifier of rudimentary p1, mod(r)p1, a trans-acting regulatory mutation of rudimentary. Mol. Gen. Genet. 248, 69–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

