The serine/threonine kinase cyclin G-associated kinase regulates epidermal growth factor receptor signaling
- PMID: 15240878
- PMCID: PMC478566
- DOI: 10.1073/pnas.0403175101
The serine/threonine kinase cyclin G-associated kinase regulates epidermal growth factor receptor signaling
Abstract
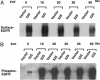
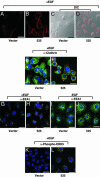
Cyclin G-associated kinase (GAK) is a serine/threonine kinase that features high homology outside its kinase domain with auxilin. Like auxilin, GAK has been shown to be a cofactor for uncoating clathrin vesicles in vitro. We investigated epidermal growth factor (EGF) receptor-mediated signaling in cells, in which GAK is down-regulated by small hairpin RNAs. Here, we report that down-regulation of GAK by small hairpin RNA has two pronounced effects on EGF receptor signaling: (i) the levels of receptor expression and tyrosine kinase activity go up by >50-fold; and (ii) the spectrum of downstream signaling is significantly changed. One very obvious result is a large increase in the levels of activated extracellular signal-regulated kinase 5 and Akt. These two effects of GAK down-regulation result from, at least in part, alterations in receptor trafficking, the most striking of which is the persistence of EGF receptor in altered cellular compartment along with activated extracellular signal-regulated kinase 5. The alterations resulting from GAK down-regulation can have distinctive biological consequences: In CV1P cells, down-regulation of GAK results in outgrowth of cells in soft agar, raising the possibility that loss of GAK function may promote tumorigenesis.
Figures



References
-
- Kanaoka, Y., Kimura, S. H., Okazaki, I., Ikeda, M. & Nojima, H. (1997) FEBS Lett. 402, 73–80. - PubMed
-
- Kimura, S. H., Tsuruga, H., Yabuta, N., Endo, Y. & Nojima, H. (1997) Genomics 44, 179–187. - PubMed
-
- Greener, T., Zhao, X., Nojima, H., Eisenberg, E. & Greene, L. E. (2000) J. Biol. Chem. 275, 1365–1370. - PubMed
-
- Umeda, A., Meyerholz, A. & Ungewickell, E. (2000) Eur. J. Cell Biol. 79, 336–342. - PubMed
-
- Korolchuk, V. I. & Banting, G. (2002) Traffic 3, 428–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

