Template-assisted filament growth by parallel stacking of tau
- PMID: 15240881
- PMCID: PMC478563
- DOI: 10.1073/pnas.0401911101
Template-assisted filament growth by parallel stacking of tau
Abstract
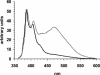
Tau filaments are found in >20 neurodegenerative diseases. Yet, because of their enormous molecular weights and poor tendency to form highly ordered 3D crystal lattices, they have evaded high-resolution structure determination. Here, we studied 25 derivatized tau mutants by using electron paramagnetic resonance and fluorescence spectroscopy to report structural details of tau filaments. Based on strong spin exchange and pyrene excimer formation of core residues, we find that individual tau proteins form single molecule layers along the fiber axis that perfectly stack on top of each other by in-register, parallel alignment of beta-strands. We suggest a model of filament growth wherein the existing filament serves as a template for the incoming, unfolded tau molecule, resulting in a new structured layer with maximized hydrogen-bonded contact surface and side-chain stacking.
Figures



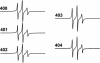

References
-
- Lee, V. M., Goedert, M. & Trojanowski, J. Q. (2001) Annu. Rev. Neurosci. 24, 1121–1159. - PubMed
-
- Goedert, M., Spillantini, M. G., Cairns, N. J. & Crowther, R. A. (1992) Neuron 8, 159–168. - PubMed
-
- Metuzals, J., Montpetit, V. & Clapin, D. F. (1981) Cell Tissue Res. 214, 455–482. - PubMed
-
- Yagishita, S., Itoh, Y., Nan, W. & Amano, N. (1981) Acta Neuropathol. 54, 239–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

