Cre-lox-regulated conditional RNA interference from transgenes
- PMID: 15240889
- PMCID: PMC478580
- DOI: 10.1073/pnas.0403954101
Cre-lox-regulated conditional RNA interference from transgenes
Abstract
We have generated two lentiviral vectors for conditional, Cre-lox-regulated, RNA interference. One vector allows for conditional activation, whereas the other permits conditional inactivation of short hairpin RNA (shRNA) expression. The former is based on a strategy in which the mouse U6 promoter has been modified by including a hybrid between a LoxP site and a TATA box. The ability to efficiently control shRNA expression by using these vectors was shown in cell-based experiments by knocking down p53, nucleophosmin and DNA methyltransferase 1. We also demonstrate the usefulness of this approach to achieve conditional, tissue-specific RNA interference in Cre-expressing transgenic mice. Combined with the growing array of Cre expression strategies, these vectors allow spatial and temporal control of shRNA expression in vivo and should facilitate functional genetic analysis in mammals.
Figures
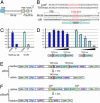
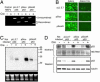

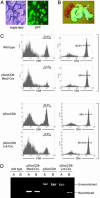
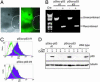
References
-
- McManus, M. T. & Sharp, P. A. (2002) Nat. Rev. Genet. 3, 737–747. - PubMed
-
- Dykxhoorn, D. M., Novina, C. D. & Sharp, P. A. (2003) Nat. Rev. Mol. Cell Biol. 4, 457–467. - PubMed
-
- Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. (1998) Annu. Rev. Biochem. 67, 227–264. - PubMed
-
- Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K. & Tuschl, T. (2001) Nature 411, 494–498. - PubMed
-
- Brummelkamp, T. R., Bernards, R. & Agami, R. (2002) Science 296, 550–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

