Lipid- and mechanosensitivities of sodium/hydrogen exchangers analyzed by electrical methods
- PMID: 15240890
- PMCID: PMC478595
- DOI: 10.1073/pnas.0403930101
Lipid- and mechanosensitivities of sodium/hydrogen exchangers analyzed by electrical methods
Abstract
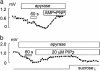
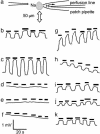
Sodium/hydrogen exchangers (NHEs) are ubiquitous ion transporters that serve multiple cell functions. We have studied two mammalian isoforms, NHE1 (ubiquitous) and NHE3 (epithelial-specific), by measuring extracellular proton (H+) gradients during whole-cell patch clamp with perfusion of the cell interior. Maximal Na(+)-dependent H+ fluxes (JH+) are equivalent to currents >20 pA for NHE1 in Chinese hamster ovary fibroblasts, >200 pA for NHE1 in guinea pig ventricular myocytes, and 5-10 pA for NHE3 in opossum kidney cells. The fluxes are blocked by an NHE inhibitor, ethylisopropylamiloride, and are absent in NHE-deficient AP-1 cells. NHE1 activity is stable with perfusion of nonhydrolyzable ATP [adenosine 5'-(beta,gamma-imido)triphosphate], is abolished by ATP depletion (2 deoxy-D-glucose with oligomycin or perfusion of apyrase), can be restored with phosphatidylinositol 4,5-bisphosphate, and is unaffected by actin cytoskeleton disruption (latrunculin or pipette perfusion of gelsolin). NHE3 (but not NHE1) is reversibly activated by phosphatidylinositol 3,4,5-trisphosphate. Both NHE1 and NHE3 activities are disrupted in giant patches during gigaohm seal formation. NHE1 (but not NHE3) is reversibly activated by cell shrinkage, even at neutral cytoplasmic pH without ATP, and inhibited by cell swelling. NHE1 in Chinese hamster ovary fibroblasts (but not NHE3 in opossum kidney cells) is inhibited by agents that thin the membrane (L-alpha-lysophosphatidylcholine and octyl-beta-D-glucopyranoside) and activated by cholesterol enrichment, which thickens membranes. Expressed in AP-1 cells, however, NHE1 is insensitive to these agents but remains sensitive to volume changes. Thus, changes of hydrophobic mismatch can modulate NHE1 but do not underlie its volume sensitivity.
Figures



References
-
- Orlowski, J. & Grinstein, S. (2003) Pflugers Arch. 447, 549–565. - PubMed
-
- Putney, L. K. & Barber, D. L. (2003) J. Biol. Chem. 278, 44645–44649. - PubMed
-
- Putney, L. K., Denker, S. P. & Barber, D. L. (2002) Annu. Rev. Pharmacol. Toxicol. 42, 527–552. - PubMed
-
- Orlowski, J. & Grinstein, S. (1997) J. Biol. Chem. 272, 22373–22376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

