Structural basis of BACH1 phosphopeptide recognition by BRCA1 tandem BRCT domains
- PMID: 15242590
- PMCID: PMC3652423
- DOI: 10.1016/j.str.2004.06.002
Structural basis of BACH1 phosphopeptide recognition by BRCA1 tandem BRCT domains
Abstract
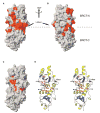
BRCT tandem domains, found in many proteins involved in DNA damage checkpoint and DNA repair pathways, were recently shown to be phosphopeptide binding motifs. Using solution nuclear magnetic resonance (NMR) spectroscopy and mutational analysis, we have characterized the interaction of BRCA1-BRCT domains with a phosphoserine-containing peptide derived from the DNA repair helicase BACH1. We show that a phenylalanine in the +3 position from the phosphoserine of BACH1 is bound to a conserved hydrophobic pocket formed between the two BRCT domains and that recognition of the phosphate group is mediated by lysine and serine side chains from the amino-terminal BRCT domain. Mutations that prevent phosphopeptide binding abolish BRCA1 function in DNA damage-induced checkpoint control. Our NMR data also reveal a dynamic interaction between BRCA1-BRCT and BACH1, where the bound phosphopeptide exists as an equilibrium of two conformations and where BRCA1-BRCT undergoes a transition to a more rigid conformation upon peptide binding.
Figures


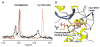

References
-
- Bork P, Hofmann K, Bucher P, Neuwald AF, Altschul SF, Koonin EV. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 1997;11:68–76. - PubMed
-
- Botuyan MVE, Mer G, Yi GS, Koth CM, Case DA, Edwards AM, Chazin WJ, Arrowsmith CH. Solution structure and dynamics of yeast elongin C in complex with a von Hippel-Lindau peptide. J Mol Biol. 2001;312:177–186. - PubMed
-
- Caldecott KW. The BRCT domain: signaling with friends? Science. 2003;302:579–580. - PubMed
-
- Callebaut I, Mornon JP. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997;400:25–30. - PubMed
-
- Cantor SB, Bell DW, Ganesan S, Kass EM, Drapkin R, Grossman S, Wahrer DC, Sgroi DC, Lane WS, Haber DA, Livingston DM. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 2001;105:149–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

