Reliability of immunoglobulin G antitoxoplasma avidity test and effects of treatment on avidity indexes of infants and pregnant women
- PMID: 15242939
- PMCID: PMC440619
- DOI: 10.1128/CDLI.11.4.669-674.2004
Reliability of immunoglobulin G antitoxoplasma avidity test and effects of treatment on avidity indexes of infants and pregnant women
Abstract
The immunoglobulin G antitoxoplasma avidity test (Vidas; BioMérieux) is an immunoenzymatic test useful for excluding acute infection after the onset of pregnancy. The avidity index (AI) is the ratio of the signal in a test sample washed with urea, which disrupts low-avidity complexes, to that washed without urea. An AI of >0.3 is taken to mean that infection had occurred more than 4 months ago. The increase of the AI with time and the influence of the different treatments given to pregnant women and their newborns were evaluated. A total of 59 pregnant women (271 sera) and their 60 neonates (199 sera) were tested from 1998 to 2002. There were five groups of women based on the type and duration of treatment given. Thirteen pregnant women (group 1) did not receive any treatment, 15 (group 2), 11 (group 3), and 17 (group 4) women received treatment with spiramycin (9 MIU/day) for 0.5 to 2, 2.5 to 5, and 5.5 to 8 months, respectively, and the last 3 women (group 5) received tritherapy (pyrimethamine-sulfonamide and spiramycin alternatively) for 1.5 to 2.5 months. All of the maternal sera collected in the first 6 months had an AI of <0.30, with a mean of 0.07 (range, 0.01 to 0.21). The increase was slow (0.02/month), and there was no significant difference when comparisons were made between the treatment groups. Neonates with proven maternofetal transmission had an increasing AI, unlike those without transmission. However, long-term therapy with pyrimethamine-sulfonamide, as opposed to treatment with spiramycin alone, was found to slow down the progression of the AI. An AI of >0.2 is sufficient to exclude acute infection in pregnant women. In neonates, it is not of major use to diagnose congenital infection; however, it could be a good indicator of compliance and efficacy of treatment of infected infants.
Figures
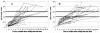


References
-
- Alvarado-Esquivel, C., S. Sethi, K. Janitschke, H. Hahn, and O. Liesenfeld. 2002. Comparison of two commercially available avidity tests for toxoplasma-specific IgG antibodies. Arch. Med. Res. 33:520-523. - PubMed
-
- Ambroise-Thomas, P., J. P. Garin, and A. Rigaud. 1966. Amélioration de la technique d'immuno-fluorescence par l'emploi de contre-colorants. Application aux toxoplasmes. Presse Med. 74:2215-2216. - PubMed
-
- Barberi, A., A. Gistri, F. Cappelletti, and I. Giordano. 2001. Diagnostic value of IgG avidity in Toxoplasma infection. Comparison of 3 commercial kits. J. Infect. Dis. 184:944-946. - PubMed
-
- Couvreur, J. 1999. Problems of congenital toxoplasmosis. Evolution over four decades. Presse Med. 28:753-757. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

