Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in sars patients by enzyme-linked immunosorbent assay
- PMID: 15243033
- PMCID: PMC446266
- DOI: 10.1128/JCM.42.7.2884-2889.2004
Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in sars patients by enzyme-linked immunosorbent assay
Abstract
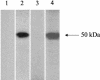
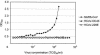
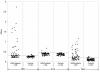
We report the development of an enzyme-linked immunosorbent assay (ELISA) for the detection of severe acute respiratory syndrome (SARS) coronavirus (CoV) nucleocapsid protein. The assay was carried out with hyperimmune polyclonal nucleocapsid-specific antibodies from guinea pigs and rabbits immunized with recombinant His(6)-tagged SARS CoV nucleocapsid protein. The assay was used for the detection of SARS CoV nucleocapsid protein in nasopharyngeal aspirate, urine, and fecal samples collected from patients with confirmed SARS between days 2 and 33 after the onset of illness. The ELISA was capable of detecting this protein in SARS CoV cell culture lysates at 15 50% tissue culture infective doses/ml but did not produce positive signals when tested with cell culture lysates of human coronaviruses OC43 and 229E. When tested with 120 nasopharyngeal aspirate, 100 urine, and 100 fecal specimens from hospitalized patients without SARS, the assay was shown to have high specificities-96.7, 99, and 96%, respectively. In an evaluation of clinical specimens from SARS patients, 34 (52%) of 66 nasopharyngeal aspirate samples from 50 patients, 5 (5%) of 94 urine samples from 94 patients, and 36 (55%) of 65 fecal samples from 65 patients tested positive for SARS CoV nucleocapsid protein. Nucleocapsid protein could be detected from days 6 to 24 in nasopharyngeal aspirate specimens, from days 11 to 31 in urine specimens, and from days 8 to 32 in fecal specimens after the onset of illness. Moreover, the protein could be detected in 25 (83%) of 30 nasopharyngeal aspirate specimens obtained from days 11 to 15 and in all 7 fecal specimens obtained from days 21 to 32. Since the present ELISA is more convenient and economical than reverse transcription-PCR, it may serve as an alternative tool for the early diagnosis of SARS CoV infection in laboratories with limited resources and expertise and for mass screening for the reservoir of SARS CoV. Further studies on serial clinical specimens should reveal the duration of nucleocapsid protein shedding and may reveal a higher detection rate in SARS patients.
Figures

References
-
- Guan, Y., B. J. Zheng, Y. Q. He, X. L. Liu, Z. X. Zhuang, C. L. Cheung, S. W. Luo, P. H. Li, L. J. Zhang, Y. J. Guan, K. M. Butt, K. L. Wong, K. W. Chan, W. Lim, K. F. Shortridge, K. Y. Yuen, J. S. Peiris, and L. L. Poon. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276-278. - PubMed
-
- Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, L. J. Anderson, et al. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. - PubMed
-
- Peiris, J. S. M., C. M. Chu, V. C. C. Cheng, K. S. Chan, I. F. N. Hung, L. L. M. Poon, K. I. Law, B. S. F. Tang, T. Y. W. Hon, C. S. Chan, K. H. Chan, J. S. C. Ng, B. J. Zheng, W. L. Ng, R. W. M. Lai, Y. Guan, K. Y. Yuen, et al. 2003. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia—a prospective study. Lancet 361:1767-1772. - PMC - PubMed
-
- Peiris, J. S. M., K. Y. Yuen, A. D. Osterhaus, and K. Stohr. 2003. The severe acute respiratory syndrome. N. Engl. J. Med. 349:2431-2441. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

