A quantitative analysis of cell volume and resting potential determination and regulation in excitable cells
- PMID: 15243134
- PMCID: PMC1665115
- DOI: 10.1113/jphysiol.2004.065706
A quantitative analysis of cell volume and resting potential determination and regulation in excitable cells
Abstract
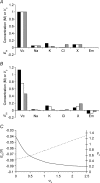
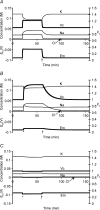
This paper quantifies recent experimental results through a general physical description of the mechanisms that might control two fundamental cellular parameters, resting potential (Em) and cell volume (Vc), thereby clarifying the complex relationships between them. Em was determined directly from a charge difference (CD) equation involving total intracellular ionic charge and membrane capacitance (Cm). This avoided the equilibrium condition dEm/dt = 0 required in determinations of Em by previous work based on the Goldman-Hodgkin-Katz equation and its derivatives and thus permitted precise calculation of Em even under non-equilibrium conditions. It could accurately model the influence upon Em of changes in Cm or Vc and of membrane transport processes such as the Na+-K+-ATPase and ion cotransport. Given a stable and adequate membrane Na+-K+-ATPase density (N), Vc and Em both converged to unique steady-state values even from sharply divergent initial intracellular ionic concentrations. For any constant set of transmembrane ion permeabilities, this set point of Vc was then determined by the intracellular membrane-impermeant solute content (X-i) and its mean charge valency (zX), while in contrast, the set point of Em was determined solely by zX. Independent changes in membrane Na+ (PNa) or K+ permeabilities (PK) or activation of cation-chloride cotransporters could perturb Vc and Em but subsequent reversal of such changes permitted full recovery of both Vc and Em to the original set points. Proportionate changes in PNa, PK and N, or changes in Cl- permeability (PCl) instead conserved steady-state Vc and Em but altered their rates of relaxation following any discrete perturbation. PCl additionally determined the relative effect of cotransporter activity on Vc and Em, in agreement with recent experimental results. In contrast, changes in Xi- produced by introduction of a finite permeability term to X- (PX) that did not alter zX caused sustained changes in Vc that were independent of Em and that persisted when PX returned to zero. Where such fluxes also altered the effective zX they additionally altered the steady state Em. This offers a basis for the suggested roles of amino acid fluxes in long-term volume regulatory processes in a variety of excitable tissues.
Figures








Similar articles
-
Membrane potential stabilization in amphibian skeletal muscle fibres in hypertonic solutions.J Physiol. 2004 Mar 1;555(Pt 2):423-38. doi: 10.1113/jphysiol.2003.058545. Epub 2003 Dec 23. J Physiol. 2004. PMID: 14694151 Free PMC article.
-
Ionic permeability of K, Na, and Cl in potassium-depolarized nerve. Dependency on pH, cooperative effects, and action of tetrodotoxin.Biophys J. 1981 Sep;35(3):677-97. doi: 10.1016/S0006-3495(81)84820-5. Biophys J. 1981. PMID: 7272457 Free PMC article.
-
Characteristics of ionic transport processes in fish intestinal epithelial cells.Biosystems. 1998 Feb;45(2):123-40. doi: 10.1016/s0303-2647(97)00071-3. Biosystems. 1998. PMID: 9544403
-
Quantitative techniques for steady-state calculation and dynamic integrated modelling of membrane potential and intracellular ion concentrations.Prog Biophys Mol Biol. 2007 Jul;94(3):336-72. doi: 10.1016/j.pbiomolbio.2006.10.001. Epub 2006 Nov 2. Prog Biophys Mol Biol. 2007. PMID: 17129600 Review.
-
The Na-K-Cl cotransporters.J Bioenerg Biomembr. 1998 Apr;30(2):161-72. doi: 10.1023/a:1020521308985. J Bioenerg Biomembr. 1998. PMID: 9672238 Review.
Cited by
-
Ionic mechanism of ouabain-induced swelling of leech Retzius neurons.Pflugers Arch. 2006 Apr;452(1):25-35. doi: 10.1007/s00424-005-0009-6. Epub 2005 Dec 10. Pflugers Arch. 2006. PMID: 16341876
-
Murine Electrophysiological Models of Cardiac Arrhythmogenesis.Physiol Rev. 2017 Jan;97(1):283-409. doi: 10.1152/physrev.00007.2016. Physiol Rev. 2017. PMID: 27974512 Free PMC article. Review.
-
Alterations in triad ultrastructure following repetitive stimulation and intracellular changes associated with exercise in amphibian skeletal muscle.J Muscle Res Cell Motil. 2007;28(1):19-28. doi: 10.1007/s10974-007-9100-2. Epub 2007 Mar 2. J Muscle Res Cell Motil. 2007. PMID: 17333488 Free PMC article.
-
How Cells Can Control Their Size by Pumping Ions.Front Cell Dev Biol. 2017 May 8;5:41. doi: 10.3389/fcell.2017.00041. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 28534026 Free PMC article.
-
Slow volume transients in amphibian skeletal muscle fibres studied in hypotonic solutions.J Physiol. 2005 Apr 1;564(Pt 1):51-63. doi: 10.1113/jphysiol.2004.080911. Epub 2005 Jan 13. J Physiol. 2005. PMID: 15649980 Free PMC article.
References
-
- Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol. 2003;82:25–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

