Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes
- PMID: 15243138
- PMCID: PMC1665128
- DOI: 10.1113/jphysiol.2004.068213
Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes
Abstract
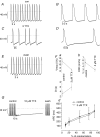
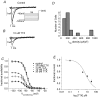
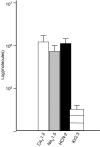
Human embryonic stem cell-derived cardiomyocytes (hES-CMs) are thought to recapitulate the embryonic development of heart cells. Given the exciting potential of hES-CMs as replacement tissue in diseased hearts, we investigated the pharmacological sensitivity and ionic current of mid-stage hES-CMs (20-35 days post plating). A high-resolution microelectrode array was used to assess conduction in multicellular preparations of hES-CMs in spontaneously contracting embryoid bodies (EBs). TTX (10 microm) dramatically slowed conduction velocity from 5.1 to 3.2 cm s(-1) while 100 microm TTX caused complete cessation of spontaneous electrical activity in all EBs studied. In contrast, the Ca2+channel blockers nifedipine or diltiazem (1 microm) had a negligible effect on conduction. These results suggested a prominent Na+ channel current, and therefore we patch-clamped isolated cells to record Na+ current and action potentials (APs). We found for isolated hES-CMs a prominent Na+ current (244 +/- 42 pA pF(-1) at 0 mV; n=19), and a hyperpolarization-activated current (HCN), but no inward rectifier K+ current. In cell clusters, 3 microm TTX induced longer AP interpulse intervals and 10 microm TTX caused cessation of spontaneous APs. In contrast nifedipine (Ca2+ channel block) and 2 mm Cs+ (HCN complete block) induced shorter AP interpulse intervals. In single cells, APs stimulated by current pulses had a maximum upstroke velocity (dV/dtmax) of 118 +/- 14 V s(-1) in control conditions; in contrast, partial block of Na+ current significantly reduced stimulated dV/dtmax (38 +/- 15 V s(-1)). RT-PCR revealed NaV1.5, CaV1.2, and HCN-2 expression but we could not detect Kir2.1. We conclude that hES-CMs at mid-range development express prominent Na+ current. The absence of background K+ current creates conditions for spontaneous activity that is sensitive to TTX in the same range of partial block of NaV1.5; thus, the NaV1.5 Na+ channel is important for initiating spontaneous excitability in hES-derived heart cells.
Figures








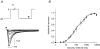
Similar articles
-
Role of sodium channels in the spontaneous excitability of early embryonic cardiomyocytes.Chin J Physiol. 2014 Aug 31;57(4):188-97. doi: 10.4077/CJP.2014.BAC202. Chin J Physiol. 2014. PMID: 25246060
-
Effects of calcium channel blockers on spontaneous electrical activity of freshly isolated three-day-old embryonic chick ventricle.Reprod Fertil Dev. 1996;8(5):921-9. doi: 10.1071/rd9960921. Reprod Fertil Dev. 1996. PMID: 8876052
-
Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization.Circ Res. 2003 Jul 11;93(1):32-9. doi: 10.1161/01.RES.0000080317.92718.99. Epub 2003 Jun 5. Circ Res. 2003. PMID: 12791707
-
Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking.J Physiol. 2004 Sep 15;559(Pt 3):835-48. doi: 10.1113/jphysiol.2004.068643. Epub 2004 Jul 14. J Physiol. 2004. PMID: 15254155 Free PMC article.
-
Mechano-Electric Feedback in the Heart: Evidence from Intracellular Microelectrode Recordings on Multicellular Preparations and Single Cells from Healthy and Diseased Tissue.In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: Academia; 2005. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: Academia; 2005. PMID: 21290768 Free Books & Documents. Review.
Cited by
-
Induced regeneration--the progress and promise of direct reprogramming for heart repair.Nat Med. 2013 Jul;19(7):829-36. doi: 10.1038/nm.3225. Nat Med. 2013. PMID: 23836233 Free PMC article. Review.
-
Genetic engineering of somatic cells to study and improve cardiac function.Europace. 2012 Nov;14 Suppl 5(Suppl 5):v40-v49. doi: 10.1093/europace/eus269. Europace. 2012. PMID: 23104914 Free PMC article.
-
Mechanisms underlying the cardiac pacemaker: the role of SK4 calcium-activated potassium channels.Acta Pharmacol Sin. 2016 Jan;37(1):82-97. doi: 10.1038/aps.2015.135. Acta Pharmacol Sin. 2016. PMID: 26725737 Free PMC article. Review.
-
De novo myocardial regeneration: advances and pitfalls.Antioxid Redox Signal. 2010 Dec 15;13(12):1867-77. doi: 10.1089/ars.2010.3388. Epub 2010 Oct 12. Antioxid Redox Signal. 2010. PMID: 20695792 Free PMC article. Review.
-
Regional difference in dynamical property of sinoatrial node pacemaking: role of na+ channel current.Biophys J. 2008 Jul;95(2):951-77. doi: 10.1529/biophysj.107.112854. Epub 2008 Apr 4. Biophys J. 2008. PMID: 18390617 Free PMC article.
References
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. - PubMed
-
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. - PubMed
-
- Cohen CJ, Bean BP, Tsien RW. Maximal upstroke velocity as an index of available sodium conductance. Comparison of maximal voltage clamp measurements of sodium current in rabbit Purkinje fibers. Circulation Res. 1984;54:636–651. - PubMed
-
- Davies MP, An RH, Doevendans P, Kubalak S, Chien KR, Kass RS. Developmental changes in ionic channel activity in the embryonic murine heart. Circ Res. 1996;78:15–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

