In search of "stemness"
- PMID: 15246154
- PMCID: PMC3279197
- DOI: 10.1016/j.exphem.2004.03.013
In search of "stemness"
Abstract
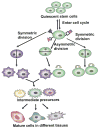
Stem cells have been identified and characterized in a variety of tissues. In this review we examine possible shared properties of stem cells. We suggest that irrespective of their lineal origin, stem cells have to respond in similar ways to regulate self-renewal and differentiation and it is likely that cell-cycle control, asymmetry/differentiation controls, cellular protective and DNA repair mechanisms, and associated apoptosis/senescence signaling pathways all might be expected to be more highly regulated in stem cells, likely by similar mechanisms. We review the literature to suggest a set of candidate stemness genes that may serve as universal stem cell markers. While we predict many similarities, we also predict that differences will exist between stem cell populations and that when transdifferentiation is considered genes expected to be both similar and different need to be examined.
Figures



References
-
- Lin H, Schagat T. Neuroblasts: a model for the asymmetric division of stem cells. Trends Genet. 1997;13:33–39. - PubMed
-
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. - PubMed
-
- Burns CE, Zon LI. Portrait of a stem cell. Dev Cell. 2002;3:612–613. - PubMed
-
- Murphy M, Bartlett PF. Molecular regulation of neural crest development. Mol Neurobiol. 1993;7:111–135. - PubMed
-
- Stanton JA, Macgregor AB, Green DP. Gene expression in the mouse preimplantation embryo. Reproduction. 2003;125:457–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

