Downregulation of prostaglandin E receptor subtype EP3 during colon cancer development
- PMID: 15247185
- PMCID: PMC1774140
- DOI: 10.1136/gut.2003.028787
Downregulation of prostaglandin E receptor subtype EP3 during colon cancer development
Abstract
Background and aims: Involvement of prostaglandin E(2) (PGE(2)) receptors EP(1), EP(2), and EP(4) in the formation of aberrant crypt foci (ACF) and/or intestinal polyps has been suggested. In contrast, EP(3) appears to have no influence on the early stages of colon carcinogenesis. In the present study, we examined expression of PGE(2) receptor subtypes EP(1), EP(2), EP(3), and EP(4) in normal colon mucosa and colon cancers, and assessed the contribution of EP(3) to colon cancer development.
Methods: mRNA expression of PGE(2) receptor subtypes EP(1), EP(2), EP(3), and EP(4) in normal colon mucosa and colon cancers in azoxymethane (AOM) treated mice and rats, and in humans, were examined by reverse transcription-polymerase chain reaction (RT-PCR), quantitative real time RT-PCR, and immunohistochemical analyses. Evaluation of the role of EP(3) was performed by intraperitoneal injection of AOM, using EP(3) receptor knockout mice. Effects of EP(3) receptor activation on cell growth of human colon cancer cell lines were examined using ONO-AE-248, an EP(3) selective agonist. Moreover, EP(3) expression in colon cancer cell lines was analysed with or without 5-aza-2'-deoxycytidine (5-aza-dC) treatment.
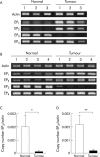
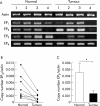
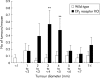
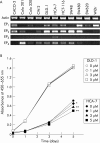
Results: Expression levels of EP(1) and EP(2) mRNA were increased in cancer tissues. EP(4) mRNA was constantly expressed in normal mucosa and cancers. In contrast, expression of EP(3) mRNA was markedly decreased in colon cancer tissues, being 5% in mice, 9% in rats, and 28% in humans compared with normal colon mucosa, analysed by quantitative real time RT-PCR. Immunohistochemical staining demonstrated the rat EP(3) receptor protein to be expressed in epithelial cells of normal mucosa and some parts of small carcinomas but hardly detectable in large carcinomas of the colon. Colon cancer development induced by AOM in EP(3) receptor knockout mice was enhanced compared with wild-type mice, with a higher incidence of colon tumours (78% v 57%) and mean number of tumours per mouse (2.17 (0.51) v 0.75 (0.15); p<0.05). Expression of EP(3) mRNA was detected in only one of 11 human colon cancer cell lines tested. Treatment with 5 microM of an EP(3) selective agonist, ONO-AE-248, resulted in a 30% decrease in viable cell numbers in the HCA-7 human colon cancer cell line in which EP(3) was expressed. Treatment with 5-aza-dC restored EP(3) expression in CACO-2, CW-2, and DLD-1 cells but not in WiDr cells, suggesting involvement of hypermethylation in the downregulation of EP(3) to some extent.
Conclusion: The PGE(2) receptor subtype EP(3) plays an important role in suppression of cell growth and its downregulation enhances colon carcinogenesis at a later stage. Hypermethylation of the EP(3) receptor gene could occur and may contribute towards downregulating EP(3) expression to some extent in colon cancers.
Figures

Similar articles
-
Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis.Cancer Res. 2002 Jan 1;62(1):28-32. Cancer Res. 2002. PMID: 11782353
-
Prostaglandin E2-induced modification of tetrodotoxin-resistant Na+ currents involves activation of both EP2 and EP4 receptors in neonatal rat nodose ganglion neurones.Br J Pharmacol. 2005 Jun;145(4):503-13. doi: 10.1038/sj.bjp.0706212. Br J Pharmacol. 2005. PMID: 15821755 Free PMC article.
-
Molecular changes in the early stage of colon carcinogenesis in rats treated with azoxymethane.J Exp Clin Cancer Res. 2002 Jun;21(2):203-11. J Exp Clin Cancer Res. 2002. PMID: 12148579
-
Prostaglandin E2 as a mediator of fever: the role of prostaglandin E (EP) receptors.Front Biosci. 2004 Sep 1;9:3046-57. doi: 10.2741/1458. Front Biosci. 2004. PMID: 15353336 Review.
-
Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents.Cancer Sci. 2004 Jun;95(6):475-80. doi: 10.1111/j.1349-7006.2004.tb03235.x. Cancer Sci. 2004. PMID: 15182426 Free PMC article. Review.
Cited by
-
Multifaceted roles of PGE2 in inflammation and cancer.Semin Immunopathol. 2013 Mar;35(2):123-37. doi: 10.1007/s00281-012-0342-8. Epub 2012 Sep 21. Semin Immunopathol. 2013. PMID: 22996682 Free PMC article. Review.
-
Microvascular COX-2/mPGES-1/EP-4 axis in human abdominal aortic aneurysm.J Lipid Res. 2013 Dec;54(12):3506-15. doi: 10.1194/jlr.M042481. Epub 2013 Oct 16. J Lipid Res. 2013. PMID: 24133193 Free PMC article.
-
Role of prostanoids in gastrointestinal cancer.J Clin Invest. 2018 Jul 2;128(7):2732-2742. doi: 10.1172/JCI97953. Epub 2018 May 7. J Clin Invest. 2018. PMID: 29733297 Free PMC article. Review.
-
Proneoplastic effects of PGE2 mediated by EP4 receptor in colorectal cancer.BMC Cancer. 2009 Jun 26;9:207. doi: 10.1186/1471-2407-9-207. BMC Cancer. 2009. PMID: 19558693 Free PMC article.
-
EP3 (prostaglandin E2 receptor 3) expression is a prognostic factor for progression-free and overall survival in sporadic breast cancer.BMC Cancer. 2018 Apr 16;18(1):431. doi: 10.1186/s12885-018-4286-9. BMC Cancer. 2018. PMID: 29661238 Free PMC article.
References
-
- Elder DJE, Paraskeva C. COX-2 inhibitors for colorectal cancer. Nat Med 1998;4:392–3. - PubMed
-
- Reddy BS, Rao CV, Rivenson A, et al. Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis 1993;14:1493–7. - PubMed
-
- Rao CV, Rivenson A, Simi B, et al. Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal anti-inflammatory agent. Cancer Res 1995;55:1464–72. - PubMed
-
- Fukutake M , Nakatsugi S, Isoi T, et al. Suppressive effects of nimesulide, a selective inhibitor of cyclooxygenase-2, on azoxymethane-induced colon carcinogenesis in mice. Carcinogenesis 1998;19:1939–42. - PubMed
-
- Labyle D , Fischer D, Vielh P, et al. Sulindac causes regression of rectal polyp in familial adenomatous polyposis. Gastroenterology 1991;101:635–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
