Synthesis and characterization of oligonucleotides containing conformationally constrained bicyclo[3.1.0]hexane pseudosugar analogs
- PMID: 15247346
- PMCID: PMC484163
- DOI: 10.1093/nar/gkh667
Synthesis and characterization of oligonucleotides containing conformationally constrained bicyclo[3.1.0]hexane pseudosugar analogs
Abstract
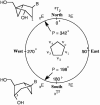
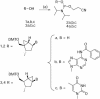
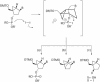
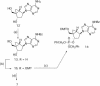
Oligodeoxyribonucleotides containing pseudorotationally locked sites derived from bicyclo[3.1.0]hexane pseudosugars have been synthesized using adenosine, thymidine and abasic versions of North- and South-methanocarba nucleosides. The reaction conditions for coupling and oxidation steps of oligonucleotide synthesis have been investigated and optimized to allow efficient and facile solid-phase synthesis using phosphoramidite chemistry. Our studies demonstrate that the use of iodine for P(III) to P(V) oxidation leads to strand cleavage at the sites where the pseudosugar is North. In contrast, the same cleavage reaction was not observed in the case of South pseudosugars. Iodine oxidation generates a 5'-phosphate oligonucleotide fragment on the resin and releases the North pseudosugar into the solution. This side reaction, which is responsible for the extremely low yields observed for the incorporation of the North pseudosugar analogs, has been studied in detail and can be easily overcome by replacing iodine with t-butylhydroperoxide as oxidant.
Figures






Similar articles
-
Biophysical studies of DNA modified with conformationally constrained nucleotides: comparison of 2'-exo (north) and 3'-exo (south) 'locked' templates.Nucleic Acids Res. 2007;35(6):1978-91. doi: 10.1093/nar/gkm025. Epub 2007 Mar 6. Nucleic Acids Res. 2007. PMID: 17341464 Free PMC article.
-
Inhibition of (cytosine C5)-methyltransferase by oligonucleotides containing flexible (cyclopentane) and conformationally constrained (bicyclo[3.1.0]hexane) abasic sites.Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):451-9. doi: 10.1081/NCN-100002319. Nucleosides Nucleotides Nucleic Acids. 2001. PMID: 11563060 Review.
-
Enantioselective synthesis of bicyclo[3.1.0]hexane carbocyclic nucleosides via a lipase-catalyzed asymmetric acetylation. Characterization of an unusual acetal byproduct.J Org Chem. 2002 Aug 23;67(17):5938-45. doi: 10.1021/jo020249u. J Org Chem. 2002. PMID: 12182625
-
Synthesis of a North-methanocarba-thymidine (N-MCT) analog.Curr Protoc Nucleic Acid Chem. 2012 Dec;Chapter 1:Unit1.29. doi: 10.1002/0471142700.nc0129s51. Curr Protoc Nucleic Acid Chem. 2012. PMID: 23255201 Free PMC article.
-
1,4:3,6-dianhydrohexitols.Adv Carbohydr Chem Biochem. 1991;49:93-173. doi: 10.1016/s0065-2318(08)60182-1. Adv Carbohydr Chem Biochem. 1991. PMID: 1814176 Review. No abstract available.
Cited by
-
Biophysical studies of DNA modified with conformationally constrained nucleotides: comparison of 2'-exo (north) and 3'-exo (south) 'locked' templates.Nucleic Acids Res. 2007;35(6):1978-91. doi: 10.1093/nar/gkm025. Epub 2007 Mar 6. Nucleic Acids Res. 2007. PMID: 17341464 Free PMC article.
-
The conformationally constrained N-methanocarba-dT analogue adopts an unexpected C4'-exo sugar pucker in the structure of a DNA hairpin.Biochemistry. 2012 Apr 3;51(13):2639-41. doi: 10.1021/bi300215k. Epub 2012 Mar 20. Biochemistry. 2012. PMID: 22409313 Free PMC article.
-
The effect on quadruplex stability of North-nucleoside derivatives in the loops of the thrombin-binding aptamer.Bioorg Med Chem. 2012 Jul 15;20(14):4186-93. doi: 10.1016/j.bmc.2012.06.005. Epub 2012 Jun 9. Bioorg Med Chem. 2012. PMID: 22727781 Free PMC article.
-
Impact of Excited-State Antiaromaticity Relief in a Fundamental Benzene Photoreaction Leading to Substituted Bicyclo[3.1.0]hexenes.J Am Chem Soc. 2020 Jun 24;142(25):10942-10954. doi: 10.1021/jacs.9b13769. Epub 2020 Jun 11. J Am Chem Soc. 2020. PMID: 32456426 Free PMC article.
-
Coupling between conformational dynamics and catalytic function at the active site of the lead-dependent ribozyme.RNA. 2018 Nov;24(11):1542-1554. doi: 10.1261/rna.067579.118. Epub 2018 Aug 15. RNA. 2018. PMID: 30111534 Free PMC article.
References
-
- Neidle S. (2000) Nucleic Acid Structure and Recognition. Oxford University Press, Oxford, New York, Chapter 5.
-
- Dickerson R.E. and Chiu,T.K. (1998) Helix bending as a factor in protein/DNA recognition. Biopolymers, 44, 361–403. - PubMed
-
- Kamath S., Sarma,M.H., Zhurkin,V.B., Turner,C.J. and Sarma,R.H. (2000) In Proceedings of the Eleventh Conversation, University of Albany, SUNY, June 15–29, 1999. J. Biomol. Struct. Dyn., 11, 317–325.
-
- Sinden R.R. (1994) DNA Structure and Function. Academic Press, San Diego, New York, Boston, Chapter 2.
-
- Saenger W. (1984) Principles of Nucleic Acid Structure. Springer-Verlag, New York, Berlin, Heilderberg, Tokyo, Chapter 9.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

