Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles
- PMID: 15247369
- PMCID: PMC519085
- DOI: 10.1104/pp.104.041723
Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles
Abstract
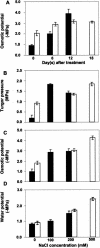
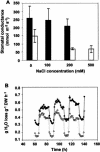
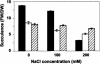
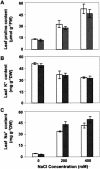
Salt cress (Thellungiella halophila) is a small winter annual crucifer with a short life cycle. It has a small genome (about 2 x Arabidopsis) with high sequence identity (average 92%) with Arabidopsis, and can be genetically transformed by the simple floral dip procedure. It is capable of copious seed production. Salt cress is an extremophile native to harsh environments and can reproduce after exposure to extreme salinity (500 mm NaCl) or cold to -15 degrees C. It is a typical halophyte that accumulates NaCl at controlled rates and also dramatic levels of Pro (>150 mm) during exposure to high salinity. Stomata of salt cress are distributed on the leaf surface at higher density, but are less open than the stomata of Arabidopsis and respond to salt stress by closing more tightly. Leaves of salt cress are more succulent-like, have a second layer of palisade mesophyll cells, and are frequently shed during extreme salt stress. Roots of salt cress develop both an extra endodermis and cortex cell layer compared to Arabidopsis. Salt cress, although salt and cold tolerant, is not exceptionally tolerant of soil desiccation. We have isolated several ethyl methanesulfonate mutants of salt cress that have reduced salinity tolerance, which provide evidence that salt tolerance in this halophyte can be significantly affected by individual genetic loci. Analysis of salt cress expressed sequence tags provides evidence for the presence of paralogs, missing in the Arabidopsis genome, and for genes with abiotic stress-relevant functions. Hybridizations of salt cress RNA targets to an Arabidopsis whole-genome oligonucleotide array indicate that commonly stress-associated transcripts are expressed at a noticeably higher level in unstressed salt cress plants and are induced rapidly under stress. Efficient transformation of salt cress allows for simple gene exchange between Arabidopsis and salt cress. In addition, the generation of T-DNA-tagged mutant collections of salt cress, already in progress, will open the door to a new era of forward and reverse genetic studies of extremophile plant biology.
Figures








References
-
- Adams P, Nelson DE, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H (1998) Growth and development of Mesembryanthemum crystallinum (Aizoaceae). New Phytol 138: 171–190 - PubMed
-
- Al-Shehbaz IA, O'Kane SL Jr, Price RA (1999) Generic placement of species excluded from Arabidopsis (Brassicaceae). Novon 9: 296–307
-
- Andras SC, Hartman TP, Marshall JA, Marchant R, Power JB, Cocking EC, Davey MR (1999) A drop-spreading technique to produce cytoplasm-free mitotic preparations from plants with small chromosomes. Chromosome Res 7: 641–647 - PubMed
-
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

