Loss of highly branched arabinans and debranching of rhamnogalacturonan I accompany loss of firm texture and cell separation during prolonged storage of apple
- PMID: 15247384
- PMCID: PMC519049
- DOI: 10.1104/pp.104.043679
Loss of highly branched arabinans and debranching of rhamnogalacturonan I accompany loss of firm texture and cell separation during prolonged storage of apple
Abstract
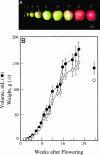
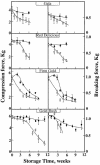
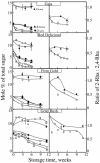
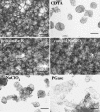
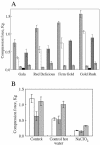
Growth and maturation of the edible cortical cells of apples (Malus domestica Borkh) are accompanied by a selective loss of pectin-associated (1-->4)-beta-D-galactan from the cell walls, whereas a selective loss of highly branched (1-->5)-alpha-L-arabinans occurs after ripening and in advance of the loss of firm texture. The selective loss of highly branched arabinans occurs during the overripening of apples of four cultivars (Gala, Red Delicious, Firm Gold, and Gold Rush) that varied markedly in storage life, but, in all instances, the loss prestages the loss of firm texture, measured by both breaking strength and compression resistance. The unbranched (1-->5)-linked arabinans remain associated with the major pectic polymer, rhamnogalacturonan I, and their content remains essentially unchanged during overripening. However, the degree of rhamnogalacturonan I branching at the rhamnosyl residues also decreases, but only after extensive loss of the highly branched arabinans. In contrast to the decrease in arabinan content, the loss of the rhamnogalacturonan I branching is tightly correlated with loss of firm texture in all cultivars, regardless of storage time. In vitro cell separation assays show that structural proteins, perhaps via their phenolic residues, and homogalacturonans also contribute to cell adhesion. Implications of these cell wall modifications in the mechanisms of apple cortex textural changes and cell separation are discussed.
Figures

Similar articles
-
Solid-state 13C NMR study of the mobility of polysaccharides in the cell walls of two apple cultivars of different firmness.Carbohydr Res. 2014 Mar 11;386:1-6. doi: 10.1016/j.carres.2013.12.019. Epub 2014 Jan 3. Carbohydr Res. 2014. PMID: 24423413
-
Cell type-specific gene expression underpins remodelling of cell wall pectin in exocarp and cortex during apple fruit development.J Exp Bot. 2019 Nov 18;70(21):6085-6099. doi: 10.1093/jxb/erz370. J Exp Bot. 2019. PMID: 31408160
-
The effects of sous-vide cooking parameters on texture and cell wall modifications in two apple cultivars: A response surface methodology approach.Food Sci Technol Int. 2017 Mar;23(2):99-109. doi: 10.1177/1082013216659197. Epub 2016 Jul 18. Food Sci Technol Int. 2017. PMID: 27413015
-
Polypotency of the immunomodulatory effect of pectins.Biochemistry (Mosc). 2013 Jul;78(7):823-35. doi: 10.1134/S0006297913070134. Biochemistry (Mosc). 2013. PMID: 24010844 Review.
-
Tissue-specific rhamnogalacturonan I forms the gel with hyperelastic properties.Biochemistry (Mosc). 2015 Jul;80(7):915-24. doi: 10.1134/S000629791507010X. Biochemistry (Mosc). 2015. PMID: 26542004 Review.
Cited by
-
Detailed Structural Characterization of Arabinans and Galactans of 14 Apple Cultivars Before and After Cold Storage.Front Plant Sci. 2018 Oct 2;9:1451. doi: 10.3389/fpls.2018.01451. eCollection 2018. Front Plant Sci. 2018. PMID: 30333848 Free PMC article.
-
Structural Characterization of Pectic Polysaccharides in the Cell Wall of Stevens Variety Cranberry Using Highly Specific Pectin-Hydrolyzing Enzymes.Polymers (Basel). 2021 Jun 2;13(11):1842. doi: 10.3390/polym13111842. Polymers (Basel). 2021. PMID: 34199419 Free PMC article.
-
Polymerization of the backbone of the pectic polysaccharide rhamnogalacturonan I.Nat Plants. 2022 Nov;8(11):1289-1303. doi: 10.1038/s41477-022-01270-3. Epub 2022 Nov 10. Nat Plants. 2022. PMID: 36357524 Free PMC article.
-
Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage.Plant Physiol. 2008 Sep;148(1):132-41. doi: 10.1104/pp.108.123513. Epub 2008 Jul 30. Plant Physiol. 2008. PMID: 18667723 Free PMC article.
-
A Glycosyltransferase from Nicotiana alata Pollen Mediates Synthesis of a Linear (1,5)-α-L-Arabinan When Expressed in Arabidopsis.Plant Physiol. 2016 Apr;170(4):1962-74. doi: 10.1104/pp.15.02005. Epub 2016 Feb 5. Plant Physiol. 2016. PMID: 26850276 Free PMC article.
References
-
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 - PubMed
-
- Aspinall GO, Fanous HK (1984) Structural investigations on the non-starchy polysaccharides of apples. Carbohyd Polym 4: 193–214
-
- Brett CT, Waldron KW (1996) Physiology and Biochemistry of Plant Cell Walls, Ed 2. Chapman and Hall, London
-
- Brummel DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47: 311–340 - PubMed
-
- Bush MS, Marry M, Huxham IM, Jarvis MC, McCann MC (2001) Developmental regulation of pectic epitopes during potato tuberisation. Planta 213: 869–880 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

