RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants
- PMID: 15247386
- PMCID: PMC519045
- DOI: 10.1104/pp.104.043927
RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants
Abstract
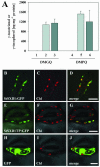
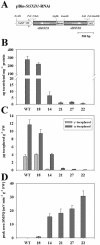
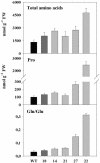
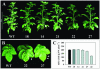
Tocopherols (vitamin E) are lipophilic antioxidants presumed to play a key role in protecting chloroplast membranes and the photosynthetic apparatus from photooxidative damage. Additional nonantioxidant functions of tocopherols have been proposed after the recent finding that the Suc export defective1 maize (Zea mays) mutant (sxd1) carries a defect in tocopherol cyclase (TC) and thus is devoid of tocopherols. However, the corresponding vitamin E deficient1 Arabidopsis mutant (vte1) lacks a phenotype analogous to sxd1, suggesting differences in tocopherol function between C4 and C3 plants. Therefore, in this study, the potato (Solanum tuberosum) ortholog of SXD1 was isolated and functionally characterized. StSXD1 encoded a protein with high TC activity in vitro, and chloroplastic localization was demonstrated by transient expression of green fluorescent protein-tagged fusion constructs. RNAi-mediated silencing of StSXD1 in transgenic potato plants resulted in the disruption of TC activity and severe tocopherol deficiency similar to the orthologous sxd1 and vte1 mutants. The nearly complete absence of tocopherols caused a characteristic photoassimilate export-defective phenotype comparable to sxd1, which appeared to be a consequence of vascular-specific callose deposition observed in source leaves. CO2 assimilation rates and photosynthetic gene expression were decreased in source leaves in close correlation with excess sugar accumulation, suggesting a carbohydrate-mediated feedback inhibition rather than a direct impact of tocopherol deficiency on photosynthetic capacity. This conclusion is further supported by an increased photosynthetic capacity of young leaves regardless of decreased tocopherol levels. Our data provide evidence that tocopherol deficiency leads to impaired photoassimilate export from source leaves in both monocot and dicot plant species and suggest significant differences among C3 plants in response to tocopherol reduction.
Figures



References
-
- Abraham E, Rigo G, Szekely G, Nagy R, Koncz C, Szabados L (2003) Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol Biol 51: 363–372 - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 - PubMed
-
- Azzi A, Ricciarelli R, Zingg JM (2002) Non-antioxidant molecular functions of α-tocopherol (vitamin E). FEBS Lett 519: 8–10 - PubMed
-
- Bergmüller E, Porfirova S, Dörmann P (2003) Characterization of an Arabidopsis mutant deficient in γ-tocopherol methyltransferase. Plant Mol Biol 52: 1181–1190 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

