Vasorin, a transforming growth factor beta-binding protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo
- PMID: 15247411
- PMCID: PMC490003
- DOI: 10.1073/pnas.0404117101
Vasorin, a transforming growth factor beta-binding protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo
Abstract
Growth factors, cell-surface receptors, adhesion molecules, and extracellular matrix proteins play critical roles in vascular pathophysiology by affecting growth, migration, differentiation, and survival of vascular cells. In a search for secreted and cell-surface molecules expressed in the cardiovascular system, by using a retrovirus-mediated signal sequence trap method, we isolated a cell-surface protein named vasorin. Vasorin is a typical type I membrane protein, containing tandem arrays of a characteristic leucine-rich repeat motif, an epidermal growth factor-like motif, and a fibronectin type III-like motif at the extracellular domain. Expression analyses demonstrated that vasorin is predominantly expressed in vascular smooth muscle cells, and that its expression is developmentally regulated. To clarify biological functions of vasorin, we searched for its binding partners and found that vasorin directly binds to transforming growth factor (TGF)-beta and attenuates TGF-beta signaling in vitro. Vasorin expression was down-regulated during vessel repair after arterial injury, and reversal of vasorin down-regulation, by using adenovirus-mediated in vivo gene transfer, significantly diminished injury-induced vascular lesion formation, at least in part, by inhibiting TGF-beta signaling in vivo. These results suggest that down-regulation of vasorin expression contributes to neointimal formation after vascular injury and that vasorin modulates cellular responses to pathological stimuli in the vessel wall. Thus, vasorin is a potential therapeutic target for vascular fibroproliferative disorders.
Figures

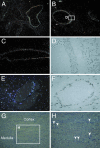
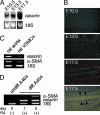

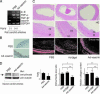
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

