NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment
- PMID: 15247420
- PMCID: PMC489993
- DOI: 10.1073/pnas.0403139101
NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment
Abstract
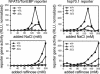
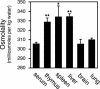
Osmotic stress responses are critical not only to the survival of unicellular organisms but also to the normal function of the mammalian kidney. However, the extent to which cells outside the kidney rely on osmotic stress responses in vivo remains unknown. Nuclear factor of activated T cells 5 (NFAT5)/tonicity enhancer binding protein (TonEBP), the only known osmosensitive mammalian transcription factor, is expressed most abundantly in the thymus and is induced upon lymphocyte activation. Here we report that NFAT5/TonEBP is not only essential for normal cell proliferation under hyperosmotic conditions but also necessary for optimal adaptive immunity. Targeted deletion of exons 6 and 7 of the Nfat5 gene, which encode a critical region of the DNA-binding domain, gave rise to a complete loss of function in the homozygous state and a partial loss of function in the heterozygous state. Complete loss of function resulted in late gestational lethality. Furthermore, hypertonicity-induced NFAT5/TonEBP transcriptional activity and hsp70.1 promoter function were completely eliminated, and cell proliferation under hyperosmotic culture conditions was markedly impaired. Partial loss of NFAT5/TonEBP function resulted in lymphoid hypocellularity and impaired antigen-specific antibody responses in viable heterozygous animals. In addition, lymphocyte proliferation ex vivo was reduced under hypertonic, but not isotonic, culture conditions. Direct measurement of tissue osmolality further revealed lymphoid tissues to be hyperosmolar. These results indicate that lymphocyte-mediated immunity is contingent on adaptation to physiologic osmotic stress, thus providing insight into the lymphoid microenvironment and the importance of the NFAT5/TonEBP osmotic stress response pathway in vivo.
Figures
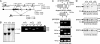


References
-
- Wood, J. M., Bremer, E., Csonka, L. N., Kraemer, R., Poolman, B., van der Heide, T. & Smith, L. T. (2001) Comp. Biochem. Physiol. A Physiol. 130, 437–460. - PubMed
-
- Yancey, P. H., Clark, M. E., Hand, S. C., Bowlus, R. D. & Somero, G. N. (1982) Science 217, 1214–1222. - PubMed
-
- Burg, M. B. (1995) Am. J. Physiol. 268, F983–F996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

